In situ architecture of the ciliary base reveals the stepwise assembly of IFT trains
Preprint posted on 15 November 2021 https://www.biorxiv.org/content/10.1101/2021.10.17.464685v1
All aboard! IFT trains assemble in a sequential manner at the ciliary base prior to entry
Selected by Nicola StevensonCategories: cell biology
Background
Cilia are microtubule-based antenna-like organelles that extend out from the surface of the cell. Here they detect biochemical and mechanical cues and, in the case of motile cilia, beat to provide motility. The main structural component of a cilium is the axoneme; a cylindrical array of 9 microtubule doublets which extends from the triplet microtubules of the mother centriole to form a protrusion. This structure is enveloped by a specialised domain of the plasma membrane, the ciliary membrane, which wraps around the microtubules and anchors to the mother centriole at the base of the cilium. This anchorage point forms the transition zone, which is a selective gate formed of multiple protein complexes that controls the entry of proteins and lipids into the cilium.
The transition zone is vital for maintaining a compartment with a molecular identity distinct from that of the cytosol or plasma membrane. Its presence, however, poses a problem for proteins with a legitimate need to access the ciliary space. The exact mechanism of transition zone selectivity and function remains elusive, however one way to pass through is to hitch a ride on an intraflagellar transport (IFT) train. These trains are composed of long linear arrays of IFT particles which use the axoneme as a track to transport cargo into and out of the cilium. There are two types of IFT particle, IFTA and IFTB, which are themselves multi-subunit assemblies. Anterograde transport towards the ciliary tip is mediated by IFTB particles, which recruit the microtubule motor kinesin-2, whilst retrograde transport is mediated by IFTA particles and the dynein-2 (dynein-1b in Chlamydomonas reinhardtii) motor.
Whilst a lot is known about the structure of the assembled IFT train and its dynamics on the axoneme, the mechanisms of assembly, loading and unloading of trains remain enigmatic. The area around the ciliary base is a crowded place with centriolar and ciliary proteins all vying for space. Copious studies show that ciliary cargo, motors and IFT proteins all accumulate here waiting for their ticket through the gate. How then is order derived from this chaos to regulate ciliary entry? In this preprint, van den Hoek et al. make a big step towards understanding this process using cryo-electron tomography (ET) and expansion microscopy to visualise IFT train assembly.
Key findings
In this study, the authors use the model organism Chlamydomonas reinhardtii, which has a readily accessible flagellum (motile cilium), to investigate IFT assembly at the ciliary base. First, they look at the structure of the transition zone itself using cryo-ET and subtomogram averaging. Y-links are known to be a key structure within the transition zone, sitting between the axoneme and ciliary membrane. In this study, the authors show that membrane binding is not a pre-requisite for Y-link formation. Stellate fibres are also apparent, forming a cross-sectional 9-pointed star within the lumen of the axoneme. Distal to these structures a previously unseen helical ‘sleeve’ was observed decorating the microtubules, which the authors predict might designate sites of axoneme severing.
Intriguingly, filamentous strings of particles were also observed. These were tethered to the transition zone at one end, with the other end extending into the cytosol. Comparison with previous structures of mature axonemal trains confirmed that these strings were assembling IFT trains. Assembling trains were more flexible than their mature axonemal counterparts and contained an extra density on IFTB near the kinesin-2 binding site of unknown identity.
Comparison of the spatial arrangement of the various IFT components revealed that trains are assembled in a sequential manner. The most complete regions of the train are adjacent to the transition zone, then as it extends into the cytosol, first the dynein-1b and then the IFTA densities are missing. This suggests the IFTB backbone is built first, followed by IFTA and then dynein-2 recruitment. Kinesin-2 is relatively small and flexible and so could not be identified by cryo-ET. Instead, the authors use expansion microscopy to show that this is the final component recruited.
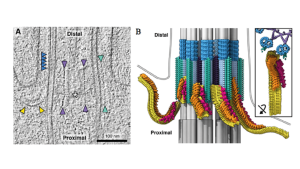
The transition zone and assembling IFT trains. Left: cryo-ET image taken from preprint figure 1. Right: Representative tomogram taken from preprint figure 2: yellow = IFTB; orange = IFTA; red = dynein1b; purple = stellate fibers; turquoise = Y-links; dark blue = MTD helical sleeve; grey = microtubules (added schematic).
Importance
I chose this preprint because it beautifully visualises the mysterious early stages of IFT train assembly. The events occurring at the basal body prior to cilium entry are essential in regulating cilium behaviour, both when it comes to building its structure and in determining its signalling capabilities, yet we know so little about them. Elucidating the mechanism of IFT train assembly helps shed light on how IFT is regulated to balance trafficking and ensure that only functional trains can take up valuable space on the axonemal highway. It is also a key step towards unravelling how the transition zone can function as a selective gate. The importance of all of these processes is evidenced by the devastating consequences of mutations in genes encoding IFT and transition zone proteins, which lead to a set of pleiotropic diseases termed ‘ciliopathies’.
Posted on: 15 November 2021
doi: https://doi.org/10.1242/prelights.31030
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (1 votes)
(1 votes)