Expansion microscopy at one nanometer resolution
Preprint posted on 5 August 2022 https://www.biorxiv.org/content/10.1101/2022.08.03.502284v1
The shape of single proteins seen with light microscopy – ONE microscopy combines the power of expansion microscopy with SRRF analysis.
Selected by Nadja HümpferCategories: biochemistry, cell biology, neuroscience
Background
Expansion microscopy (ExM) is an upcoming technique that circumvents the diffraction limit of light microscopy by physically expanding the sample [1]. In ExM, the specimen is embedded in a swellable hydrogel that expands when soaked in water. This leads to physical separation of the structures of interest and the introduced fluorophores. The factor of expansion determines the increase in resolution. Researchers have tried to combine ExM with other super-resolution imaging methods to push the resolution even further.
Shaib and colleagues are the first to introduce SRRF to 10-fold ExM. SRRF stands for super-resolution radial fluctuations and this approach benefits from a separation of fluorophores [2], as is the case in 10-fold ExM. Similarly, the background fluorescence is diluted to an almost neglectable level. Measuring the intensity fluctuations of well-separated fluorophores in almost zero-background conditions allows for super-resolution imaging based on an SRRF-algorithm. Combining the 10-fold increase in resolution due to expansion with the increased resolution due to SRRF results in a final resolution of 1 nm. At this resolution, the shape of individual proteins becomes apparent when labelled with a pan-protein staining. The authors show that their method is applicable in a broad diagnostic context.
Key findings
The shape of single proteins or protein complexes is resolved
The most exciting finding of this preprint is the visualization of purified proteins. To this end, proteins, such as antibodies, were embedded in the gel and then homogenized to allow the 10-fold expansion. In the process of gel-homogenization, the samples were digested by protease cleavage. The digestion introduced reactive amine groups that could be labelled by reacting with NHS-ester coupled dyes. An entire IgG antibody could therefore be labelled with NHS-ester coupled fluorophores. Due to the high resolution below 1 nm, the shape of the antibody became apparent. It was possible to show the architecture of antibodies of different classes. Remarkably, the authors were able to show the shape of different proteins, such as GABA-receptors and calmodulin. Moreover, the structure of the protein otoferlin, which has not been determined until now, could be resolved. This structure fitted the prediction of AlphaFold, showing the potential of ONE microscopy in the uncovering of thus far unknown protein structures.
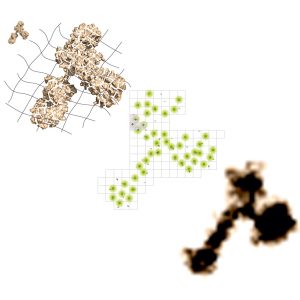
Figure 1: Schematic of the steps taken in ONE microscopy: The antibody is anchored into a X10 gel and digested. After labelling with fluorophores, the signal is detected and analysed with SRRF: The shape of the antibody becomes visible with fluorescence microscopy. (C) Rizzoli Lab
A GFP-based ruler shows the resolution is below 1 nm
To prove the resolution to be below one nanometer, the authors generated a ‘triangulate smart ruler’ (TSR). This protein complex consists of a GFP-molecule with an ALFA-tag. Fluorescently labelled nanobodies against GFP as well as the tag were incubated with the protein. After the ONE procedure, it was possible to visualize the individual fluorophores on the nanobodies. Strikingly, the architecture of a GFP molecule bound by three different nanobodies was reflected in the distribution of the fluorescence signal. Distances as low as 2 nm could be visualized, as proven by the appearance of two separate fluorophores per nanobody, 2 nm apart. Full width at half-maximum measurements showed the resolution to be below 1 nm for different fluorophores.
Applications of ONE microscopy for diagnostics
As Parkinson’s disease is characterized by the appearance of clusters or aggregates of the protein alpha-synuclein (ASYN) and ONE microscopy could impressively show the shape of individual proteins, the authors used their method to directly visualize the ASYN-clusters in samples from patients. They sampled the cerebral spinal fluid (CSF) of persons diagnosed with Parkinson’s disease and compared it to a control group. The detection of the aggregates in the CSF was dependent on a nanobody against alpha-synuclein. The increased resolution thanks to ONE microscopy enabled the detection of clusters and aggregates of different shapes and sizes. The authors found significant differences in the appearance and number of ASYN-complexes in the group diagnosed with Parkinson’s disease compared to the control group. This opens up the route of using ONE microscopy as a diagnostic tool for neurodegenerative diseases.
What I like about this work
The work impressively shows the potential of expansion microscopy when combined with other imaging (analysis) techniques. After the introduction of ExM, it was readily combined with light sheet imaging [3], single molecule localization microscopy (SMLM)[4] or stimulated emission depletion (STED) microscopy [5]. However, due to some prerequisites of the methods, it had not been possible to reach the resolution level reported in this preprint. SMLM, for example, requires blinking of fluorophores, which depends on buffer conditions. Buffers that facilitate blinking, however, contain salts that cause the expanded gel to shrink. STED on the other hand requires very strong fluorescent signal – which is difficult to achieve when samples expand and therefore signal is diluted. I found it very innovative that the authors of this preprint made use of this ‘disadvantage’ of diluted fluorophores and combined it with an imaging method that does not rely on high photon-count but instead makes use of signal-fluctuations such as SRRF.
When introducing a groundbreaking technique like this, the evaluation and control is of utmost importance. I really enjoyed the introduction of the TSR as ruler for the achievable resolution. Seeing individual fluorophores on a protein complex only a few nanometers in size was astonishing to me.
In general, the application of ONE to single proteins or protein-complexes with a pan-staining is very impressive. The results are reminiscent of single particle cryo-EM, but based on fluorescence microscopy. It was mind-blowing to see the molecular shape of a protein visualized by a light microscope. I also expect the ONE approach to be more accessible than single-particle cryo-EM, due to the availability of standard fluorescence microscopes and a public analysis pipeline.
Future directions
The introduction of ONE microscopy will be of major importance to the light microscopy field. It is a cheap alternative to other super-resolution techniques. It also provides resolution that could not be achieved previously. Therefore, it bridges the gap between light microscopy and electron microscopy. The plethora of applications, only a few covered in this preLight, speaks for a potential in different areas of both research as well as diagnostics. I envision many laboratories and researchers around the world using this technology to visualize the shape of their proteins of interest without going through the time- and resource-consuming process of crystallography or cryo-EM.
Questions for the authors
Q1: You show that a general amine-staining with fluorescein can reveal the shape of proteins. Can you explain why in your initial experiments with the TSR, the shape of the GFP barrel was not visible (Figure 1D)? It rather looks like a single fluorophore signal, which is bound to GFP.
Q2: In Figure 3, you show how the extraction of cholesterol induces spreading of synaptic molecules in the presynapse. However, you mention that synaptic vesicles stay intact and the number of synaptotagmin molecules per individual vesicle remains the same. I was wondering how it is possible to still count synaptotagmin molecules on those dispersed vesicles?
Q3: What does one need to consider when choosing the fluorophores for staining? I was particularly interested in the fluorescein molecule; as this seems to be rather unusual for fluorescent light microscopy.
Q4: How do you practically find the individual proteins in the gel? I can imagine a 10-fold expanded gel is quite difficult to handle and to look for a region of interest must take a very long time.
References
- Chen, F., P.W. Tillberg, and E.S. Boyden, Optical imaging. Expansion microscopy. Science, 2015. 347(6221): p. 543-8.
- Culley, S., et al., SRRF: Universal live-cell super-resolution microscopy. Int J Biochem Cell Biol, 2018. 101: p. 74-79.
- Burgers, J., et al., Light-sheet fluorescence expansion microscopy: fast mapping of neural circuits at super resolution. Neurophotonics, 2019. 6(1): p. 015005.
- Zwettler, F.U., et al., Molecular resolution imaging by post-labeling expansion single-molecule localization microscopy (Ex-SMLM). Nat Commun, 2020. 11(1): p. 3388. link to preLight
- Gao, M., et al., Expansion Stimulated Emission Depletion Microscopy (ExSTED). ACS Nano, 2018. 12(5): p. 4178-4185.
Posted on: 24 November 2022
doi: https://doi.org/10.1242/prelights.33191
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Structural basis of respiratory complexes adaptation to cold temperatures
Lens Placode Modulates Extracellular Matrix Formation During Early Eye Development
Generalized Biomolecular Modeling and Design with RoseTTAFold All-Atom
Also in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Also in the neuroscience category:
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
Synergistic olfactory processing for social plasticity in desert locusts
A choroid plexus apocrine secretion mechanism shapes CSF proteome and embryonic brain development
preLists in the biochemistry category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
Preprint Peer Review – Biochemistry Course at UFRJ, Brazil
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biochemistry deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (1 votes)
(1 votes)