3-Dimensional Organization and Dynamics of the Microsporidian Polar Tube Invasion Machinery
Preprint posted on 4 April 2020 https://www.biorxiv.org/content/10.1101/2020.04.03.024240v1
Article now published in PLOS Pathogens at http://dx.doi.org/10.1371/journal.ppat.1008738
Categories: biophysics, cell biology, microbiology
The Backstory
Microsporidia are unicellular, eukaryotic parasites that fire harpoon-like polar tubes to invade host cells. They infect invertebrates and vertebrates including humans, rely on the host for genome duplication and produce spores that can survive outside the host. The polar tube (PT) remains as a coiled spring-like structure in dormant spores. Once triggered by environmental cues, the PT is ejected within milliseconds as a linear tube, several times the length of the spore that latches onto the host cell and may even penetrate it, providing a channel for the delivery of the infectious cargo to the host cytoplasm, in a process termed spore germination. Though microsporidia were discovered more than a century ago, aspects of the 3-dimensional design of the PT, the mechanics of its extrusion and the transport of cargo still remain enigmatic. For instance, how the linear segment of the PT connects with the coiled region remains unclear. Further, extremely rapid time scales of the process pose a major hurdle to uncovering the kinetics of PT extrusion. To start with, the authors use serial block-face scanning electron microscopy (SBFSEM) to provide a 3D model of the PT in dormant spores, and its position relative to other organelles. Next, they follow it up with high speed live-cell microscopy (up to 50 frames/ second) to capture PT extrusion and cargo transport in real time to precisely lay out the steps involved in spore germination in microsporidia. That’s not it, the authors go a step further from investigating the process in the well-studied Anncaliia algerae, to test two more species and define universal features of microsporidians while pointing out the individualities.
Key findings
At the outset the authors describe the architecture of the A. algerae egg-shaped spore, and the packaging of the PT within it, accurately providing dimensions of the spore, number of turns in the PT coil and its tilting away from the anterior-posterior (A-P) axis. Interestingly, the PT coil always formed a right-handed helix, raising new questions about how such a bias arises and its significance to PT function. The authors go on to clarify that the linear segment of the PT is connected to the anterior end of the PT coil, ruling out the possibility that a linear PT segment runs across the entire length of the spore. In addition to the PT, the A. algerae spore carries a centrally placed anterior anchoring disc and two nuclei cradled by a cup-shaped vacuole, at the posterior end, that may be involved in PT extrusion. Consistent with previous 2D TEM images, the authors show that the spore is enclosed in an outer dense exospore layer and an inner endospore shell that is thinnest around the apical anchoring disc, creating the most feasible site for PT ejection. Are these features universal to microsporidia? Turns out, there are similarities in the general layout, with different species varying in spore morphology, spacing between the turns of the PT coil and position of the anchoring disc which was off-centred from the A-P axis in case of Encephalitozoon hellem spores.
The differences across microsporidians were not just restricted to spore organisation, but also extended to the mechanics of polar tube extrusion. As hypothesized from the position of the anchoring disc, PT ejection in A. algerae occurred centrally while that in E. hellem was off-centre. Spore germination had three phases, PT elongation, a static phase with no change in PT length, and cargo emergence at the PT tip, taking about 1.6s in A. algerae. The process is completed in E. hellem, within half a second with a maximum velocity of ~330 µm•s-1 and acceleration of ~5000 µm•s-2 and a reduced static phase. Surprisingly, fully extended PTs were double the length of PTs packed in the spores with A. algerae going over 100 µm, possibly achieved through conformational changes in proteins that build the PT. Shortening of the PT after ejection was also seen on some occasions. Invariably the process ended with the release of a circular cargo at the distal end of the PT.
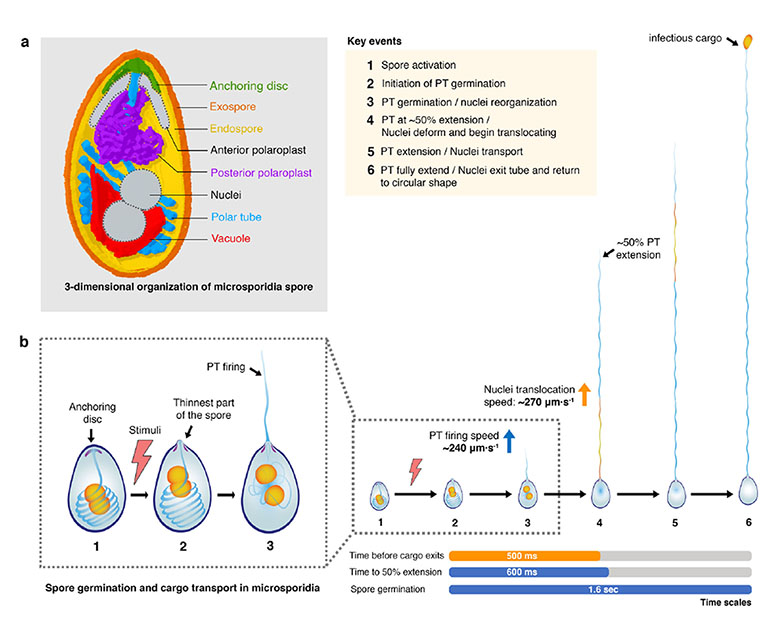
Finally, the authors turned their attention to cargo transport, the last step of the process. In absence of vast information about the nature of the cargo, and DNA being essential for proliferation within the host, the authors looked at nuclear dynamics as a representative cargo. While the nuclei went through some structural rearrangement within the spore, the nucleus entered the PT once it reached about half its length. How was a nucleus about 7 times the width of this narrow tunnel going to pass through? The nuclei displayed great plasticity within the PT, showing extreme elongation with an aspect ratio of 0.1 within the PT to emerging as a rather circular cargo outside the PT. The nuclei initially travel fast through the PT and then slow down before they are spooled into a circular cargo.
Why I like it?
Microsporidia are believed to be a highly diverged phylum of fungi. The process reminded me of germ tube extension and nuclear migration key to infection by fungal pathogens, strikingly microsporidian PT extrusion occured at phenomenally faster timescales. The fungal nuclei also display a great deal of flexibility in passing through narrow constrictions. Wonder if there are lessons that can be exchanged between the two groups? Also the preprint is detailed with spatio-temporal measurements and has really nice videos of polar tube extension and nuclear dynamics.
Questions
- How did you get interested in polar tube extrusion? Could you share the challenges and the excitement of the findings of this study?
- The egg-shaped spores of algerae have a clear apex and show centre placement of the anchoring disc. Is the lack of such morphology and the cylindrical shape of spores likely to cause off-centre positioning in E. hellem?
- How do the nuclei achieve this plasticity? Is there a breakdown of the nuclear envelope with only the DNA passing or are they travelling as intact nuclei? Do the two nuclei fuse prior to entry into and travel as one mass?
- The nuclear movement seems biphasic in algerae. How does the slow moving phase of the nucleus correlate with the pause of PT elongation? Is the slow phase of nuclear migration eliminated in case of E. hellem nuclei?
- The video of algerae PT elongation shows a wave like zigzag movement? Is the PT tip exploring the environment as it elongates?
Posted on: 19 April 2020
doi: https://doi.org/10.1242/prelights.18758
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biophysics category:
Structural basis of respiratory complexes adaptation to cold temperatures
Actin polymerization drives lumen formation in a human epiblast model
Learning a conserved mechanism for early neuroectoderm morphogenesis
Also in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Also in the microbiology category:
Digital Microbe: A Genome-Informed Data Integration Framework for Collaborative Research on Emerging Model Organisms
Mixed Alkyl/Aryl Phosphonates Identify Metabolic Serine Hydrolases as Antimalarial Targets
NAD+ metabolism is a key modulator of bacterial respiratory epithelial infections
preLists in the biophysics category:
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the microbiology category:
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)