A deeper understanding of intestinal organoid metabolism revealed by combining fluorescence lifetime imaging microscopy (FLIM) and extracellular flux analyses
Preprint posted on 16 September 2019 https://www.biorxiv.org/content/10.1101/771188v1
Article now published in Redox Biology at http://dx.doi.org/10.1016/j.redox.2019.101420
Shedding light on unique metabolic properties of the Lgr5+ stem cell niche by O2 phosphorescence & NAD(P)H fluorescence imaging of intestinal organoids.
Selected by Jessica XieCategories: cell biology
Background
To say that organoid culture technologies have revolutionized human biology and medicine in recent years is nothing short of an understatement. The discovery and development of methods to generate 3D organoids for virtually all tissues have enabled the study of self-organized complex systems at a depth previously painfully limited by accessibility of human samples. Consequently, organoids have seen use in a wide range of applications—from developmental biology to disease modeling, drug screening, personalized medicine, and even regenerative medicine.
Several challenges remain, however. For all their advantages, the very heterogeneity that distinguishes and defines organoids also renders it difficult to perform assays previously developed for homogeneous 2D cultures. Intestinal organoids, this study’s chosen model system, are especially notable among the many organoid models for their dramatic 3D spatial heterogeneity—far more than being cellular aggregations, they display clear apicobasal polarity with crypt domains containing LGR5+ stem cells, and villus domains containing differentiated enterocytes and enteroendocrine cells.
Current methods to study metabolism generally fall into either of two categories: 1) bulk methods that take extracellular measurements (most commonly of oxygen and pH) of media containing a whole organoid, or 2) disruptive methods following flow sorting of dissociated organoids. Here, the authors pioneer a third approach: microscopy-based techniques to measure oxygen (O2) and NADH/NADPH in intact intestinal organoids while preserving spatial information.
Key findings
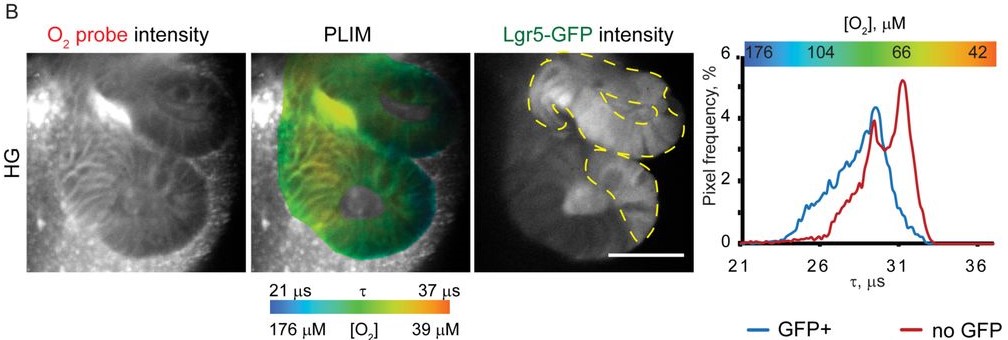
To measure oxygen levels, they use a small molecule—Pt-Glc—whose phosphorescence is quenched by oxygen. After mouse intestinal organoids are stained with the probe, oxygen levels can be determined by phosphorescence lifetime imaging microscopy (PLIM). The authors also measure fluorescence along with phosphorescence in LGR5-GFP organoids, where GFP marks the stem cell niche and non-fluorescent regions contain more differentiated cells. Using this setup, they find higher oxygenation in GFP+ stem cell-containing regions compared to GFP– differentiated regions across various culture conditions of metabolic stress (namely low glucose and pyruvate withdrawal).
Next, the authors use two-photon excitation microscopy to measure NAD(P)H autofluorescence in stem cell-containing and differentiated regions of LGR5-GFP organoids. Analysis of the fluorescence decay curves provides information on properties of NADH/NADPH: the proportion of enzyme-bound (vs free) NAD(P)H, and mean fluorescence lifetime of bound NAD(P)H. Here, the authors report a decrease in NAD(P)H lifetime in GFP+ regions compared to GFP– regions, as well as some differences in the proportion of bound NAD(P)H in GFP+ and GFP– regions across various media conditions.
As with every nascent technology, however, there were several limitations and complications: firstly, NADPH and NADH must often—as in this case—be measured together due to their very similar fluorescence properties. Secondly, only protein-bound NAD(P)H could be accurately measured, as the picosecond-range fluorescence lifetime of free NAD(P)H was too short. Additionally, autofluorescence was seen in the intestinal organoid lumen. Finally, the biological significance of changes in these properties was not entirely straightforward to determine.
Nonetheless, the authors provide evidence to indicate distinct metabolic dynamics in stem and differentiated cells within intestinal organoids, demonstrating the utility of these techniques for studying metabolism within specific regions of a complex organoid system.
Questions for the authors
- What other complementary techniques can be used to cross-validate oxygen and NAD(P)H measurements?
- Which other pathways or molecules might be most interesting to look at, alongside NAD(P)H & O2?
- You saw clear differences in O2 levels in GFP+ vs GFP– regions, but nothing as obvious for NAD(P)H—I’m curious if you would attribute that to limited sensitivity of the technique, or do you think that biological differences aren’t as large?
- Similarly, in Figure 5 you show that organoids with more distinct LGR5-GFP domains have larger differences in O2 level than more ‘mosaic’ organoids. Do you think this is due to imaging resolution/sensitivity, or biological differences (perhaps organoids with more distinct GFP domains had higher quality differentiation than organoids that became more mixed)?
Posted on: 30 October 2019
doi: https://doi.org/10.1242/prelights.14831
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)