A Rap1 binding site and lipid-dependent helix in talin F1 domain cooperate in integrin activation
Preprint posted on 21 December 2018 https://www.biorxiv.org/content/early/2018/12/21/504894
Article now published in The Journal of Cell Biology at http://dx.doi.org/10.1083/jcb.201810061
The critical Rap1 – talin binding site isn’t where we thought it was in mammals. Rap1- talin F1 binding is necessary for integrin activation.
Selected by Amanda HaageWhy This Is Cool – To understand this paper, you need a bit of background in the talin field. First, talin regulates almost all aspects of cell-ECM adhesion, including being essential for integrin activation1. Second, Rap1 has also been shown to be essential for integrin activation2, supposedly through its role in talin recruitment. The original dogma was that Rap1 recruited talin to the membrane through another adaptor protein called RIAM. The almost complete lack of phenotype in RIAM knockout mice indicated that there must be an alternative mechanism3, and people hypothesized that Rap1 could bind talin itself, independent of RIAM. Structural in vitro work4, a study in Dictyostelium5, and a study in Drosophila6 have since demonstrated that Rap1 could bind talin through its F0 domain and blocking this binding had severe consequences for morphogenesis. Although this was seen in multiple systems, the analogous talin1 F0 point mutation in mice did not lead to defects in mammalian development or integrin activation7. Here is where our authors start.
The basis of this study is the revelation that the talin head has a second Rap1 binding site in its F1 domain. Using sequence alignment and superimposition of the NMR structures, the authors identify two conserved F1 residues, R98 and R118, which are compatible with Rap1 binding. Titration data followed this up and determined weak affinity for talin F1 and Rap1. This affinity was lost when the authors introduced the R118E mutation. Expressing the single R35E and R118E mutant form of talin, as well as a double mutant in CHO A5 cells drastically decreased integrin activation. This activation could not be rescued with the expression of a constitutively active Rap1 mutant. These data created an obvious question, talin F0 and F1 have a similar affinity for Rap1, but why does only one have an impact on integrin activation? Talin F1 is unique in that it also contains an unstructured loop. Removal of this loop also drastically decreases integrin activation. This decrease cannot be rescued by the expression of a constitutively active Rap1 mutant and is not worsened by the addition of the R118E mutation. Thus, this study identifies two new mechanisms for Rap1-mediated integrin activation through the talin F1 domain.
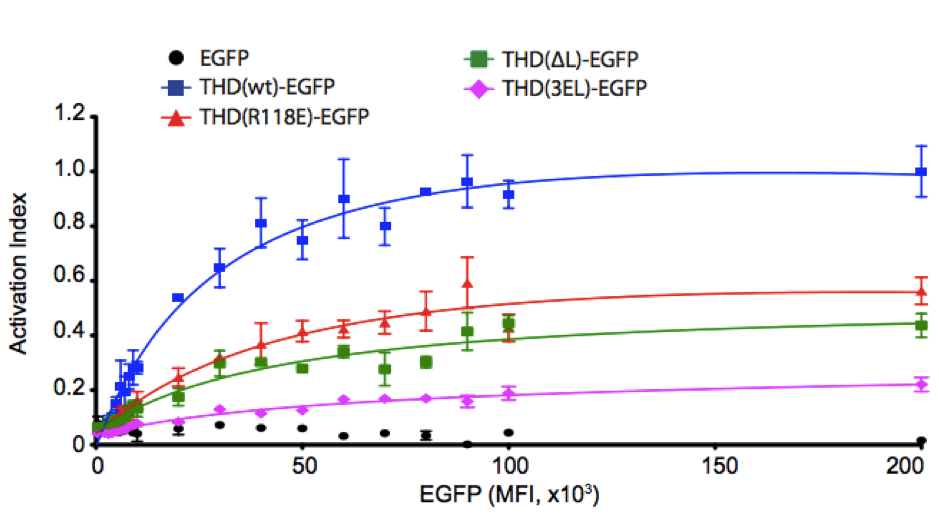
Why I Selected It – In recent years, several studies have showed the importance of Rap1 mediated talin recruitment, but the exact mechanisms in different contexts remain unclear. This study provides another important and exciting part of the puzzle. Not only does it provide an important step forward in the field, it does so in a clear and well thought-out way. This paper will be greatly appreciated by all of us who work on talin and who will be citing it in our future work.
Open Questions –
- Does the loss of the F1 loop impact Rap1 affinity?
- Is the F1 loop in talin conserved?
- How do you reconcile the inconsistent results between Dictyostelium, Drosophila, and mice about which domain (F0 or F1) is more important in direct Rap1 binding?
Related References –
- Talin is a master regulator of cell-ECM adhesion.
- Klapholz B. & Brown NH. (2017). Talin – the master of integrin adhesions. Cell Sci 130(15): 2435 – 2446.
- Rap1 is essential for integrin activation
- Stefanini L, Lee RH, Paul DS, O’Shaughnessy EC, Ghalloussi D, Jones CI, Boulaftali Y, Poe KO, Piatt R, Kechele DO, Caron KM, Hahn KM, Gibbins JM, Bergmeier W. (2018). Functional redundancy between rap1 isoforms in murine platelet production and function. Blood. 132(18): 1951-1962.
- RIAM knockout mice
- Stritt S, Wolf K, Lorenz V, Vogtle T, Gupta S, Bosl MR, Nieswandt B. (2015). Rap1-GTP-interaction adaptor molecule (RIAM) is dispensable for platelet integrin activation and function in mice. Blood. 125(2):219-222.
- Klapproth S, Sperandio M, Pinheiro EM, Prunster M, Soehnlein O, Gertler FB, Fassler R, Moser M. (2015). Loss of the rap1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice. Blood. 126(25):2704-2712.
- Rap1 F0 binding in vitro
- Goult BT, Bouaouina M, Elliott PR, Bate N, Patel B, Gingras AR, Grossmann JG, Roberts GC, Calderwood DA, Critchley DR, Barsukov IL. (2010). Structure of a double ubiquitin-like domain in the talin head: a role in integrin activation. EMBO J. 29(6):1069 – 1080.
- Rap1 F0 binding in Drosophila
- Camp D, Haage A, Solianova V, Castle WM, Xu QA, Lostchuck E, Goult BT, Tanentzapf G. (2018). Direct binding of talin to rap1 is required for cell-ECM adhesion in drosophila. Cell Sci. 131(24):225144
- Rap1 F0 binding in Dictyostelium
- Plak K, Pots H, Van Haastert PJ, Kortholt A. (2016). Direct interaction between talinB and rap1 is necessary for adhesion of dictyostelium cells. BMC Cell Biol. 17(1): doi: 10.1186/s12860-015-0078-0.
- Rap1 F0 binding in mice
- Lagarrigue F, Gingras AR, Paul DS, Valadez AJ, Cuevas MN, Sun H, Lopez-Ramirez MA, Goult BT, Shattil SJ, Bergmeier W, Ginsberg MH. (2018). Rap1 binding to the talin 1 F0 domain makes a minimal contribution to murine platelet GPIIb-IIIa activation. Blood Adv. 2(18): 2358-2368.
Posted on: 20 January 2019
doi: https://doi.org/10.1242/prelights.7673
Read preprint










 (No Ratings Yet)
(No Ratings Yet)