Aqueous synthesis of a small-molecule lanthanide chelator amenable to copper-free click chemistry
Preprint posted on 26 January 2019 https://www.biorxiv.org/content/10.1101/531087v1
Article now published in PLOS ONE at http://dx.doi.org/10.1371/journal.pone.0209726
Clicking into place: synthesising a small-molecule lanthanide chelator under aqueous conditions
Selected by Zhang-He GohCategories: biochemistry, pharmacology and toxicology, synthetic biology
Background of preprint
Lanthanides are popular tools for structure determination and analysis via nuclear magnetic resonance (NMR) by virtue of their favourable chemical properties in spectroscopy and their unique paramagnetic properties. However, this strategy requires site-specific incorporation of these lanthanides, often involving the use of a chelating moiety to rigidly orient the lanthanide. Currently, lanthanide chelators have generally been incorporated into target proteins via genetic insertion of a lanthanide binding tag (LBT), but the large size of the LBT may interfere with proper folding and even the function of the target protein. Therefore, research has turned towards the use of click chemistry to incorporate small molecule lanthanide chelators into large biomolecules instead. Because the copper catalyst frequently used in click chemistry presents problems associated with biological incompatibility, Bishop et al. aimed to invent a method to incorporate the chelator into a given protein of interest. Bishop et al. hypothesised that site-specific protein labelling could be achieved through two steps:
- Site-directed mutagenesis and incorporation of the unnatural amino acid para-azidophenylalanine (paF) into the protein of interest, and
- Use of copper-free click chemistry to adduct a cycloocyne-containing paramagnetic and fluorescent lanthanide chelator to the incorporated paF in the protein of interest.
Key findings of preprint
This preprint provides a proof-of-concept that demonstrates the feasibility of their approach to overcoming the problems currently associated with incorporating small molecule lanthanide chelators into large biomolecules. The experiments in this preprint can be divided into three sections (Fig. 1).
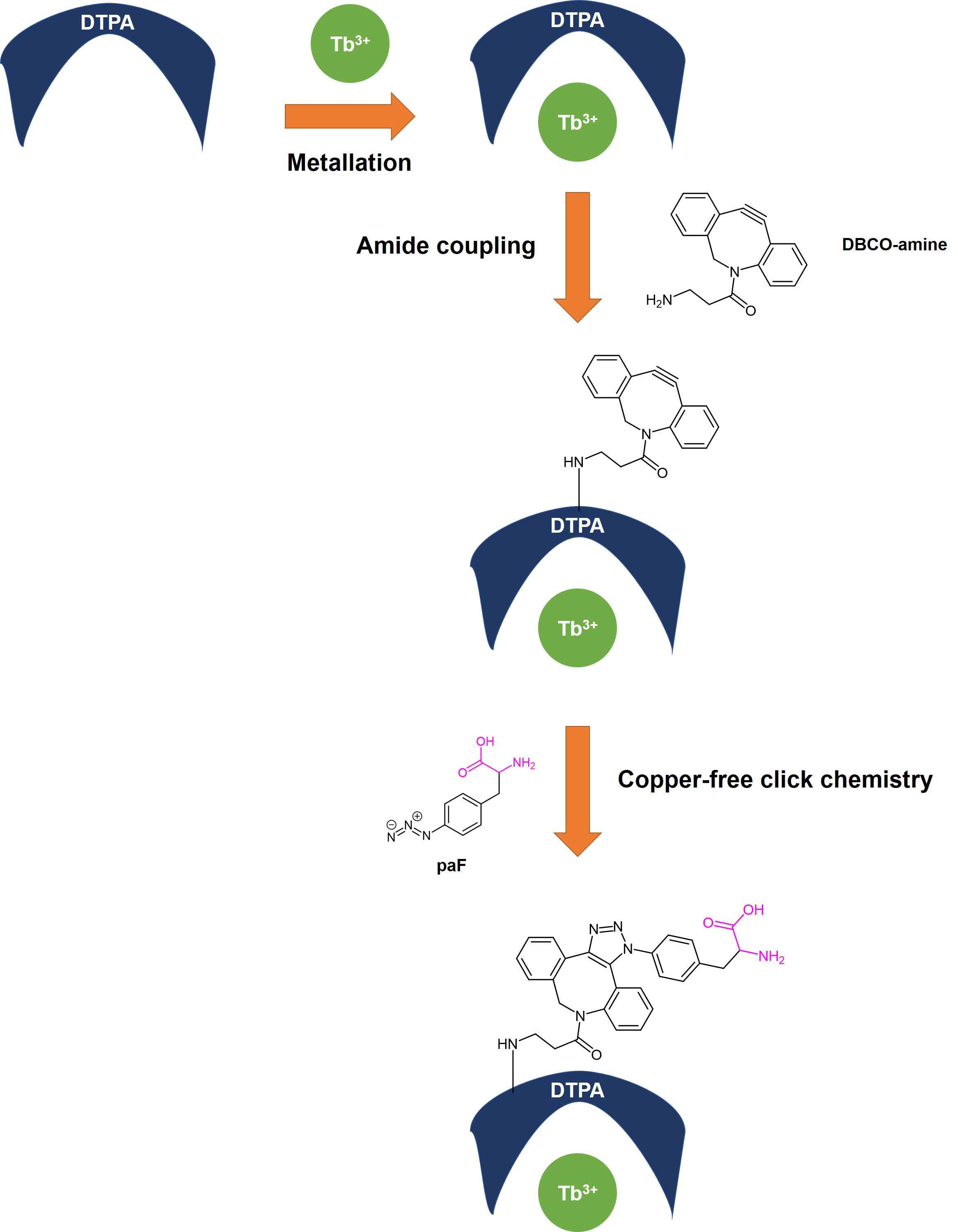
Figure 1. Outline of experiments in Bishop et al. The backbone of paF that can be incorporated into a peptide chain is in pink.
(A) Metalation of Diethylenetriamine-pentaacetic acid (DTPA)
Following their initial investigations, the authors decided to focus on terbium for four reasons: (a) its attomolar DTPA binding affinity, (b) its substantial fluorescence intensity, (c) its ability to induce pseudocontact shifts, and (d) its large paramagnetic relaxation enhancement (PRE)—an NMR technique which is commonly used in binding studies. In this step, Bishop et al. metalated DTPA with Tb3+ to generate DTPA:Tb. Two findings are especially pertinent here:
- Terbium remained chelated throughout synthesis and purification, leading the authors to posit that terbium is not displaced by bi- and tri-valent cations in subsequent click reactions; and
- Further incubation of DTPA:Tb for a longer duration did not lead to an increase in fluorescence intensity, suggesting that DTPA could chelate the majority of Tb3+ even without a lengthy incubation.
(B) Synthesis of Clickable Lanthanide Chelator (CLC) through Amide Coupling to DTPA:Tb
DTPA:Tb was then coupled with Dibenzylcyclo-octyne-amine (DBCO-amine) to generate the clickable lanthanide chelator (CLC).
(C) Copper-free Click Chemistry Reactions
Finally, copper-free Click chemistry was performed between CLC and paF to generate the clicked clickable chelator (CCC). Based on the excess of CLC used in their experiment, Bishop et al. estimated that this reaction proceeded to completion, utilising all available paF.
What I like about this preprint
I chose this preprint for two reasons. First, it exemplifies the increasingly interdisciplinary work of much scientific research today. By applying discoveries in lanthanide chemistry in the synthesis of the CLC and click chemistry in the final synthesis of the CCC, Bishop et al. illustrate how knowledge in inorganic and organic chemistry can contribute to the invention of technologies that would further advance the field of molecular biology.
Second, this preprint is exciting because its potential applications are wide-ranging. In the field of healthcare, being able to label biomolecules with novel probes can help make significant headway in diagnostic applications. In molecular biology, the use of these probes could also help researchers to better characterise biomolecules—not just proteins, but nucleic acids as well.
Future directions
Future directions will involve the development of the proof-of-concept that has been established in this preprint. In this preLight, I offer three potential ways in which the findings in this preprint may be applied.
First, other experiments will be needed to first demonstrate how the CLC can be clicked with paF-containing proteins of interest to form labelled forms of the proteins of interest. After the CLC has been shown to click to proteins successfully, researchers will then need to evaluate the utility of these probes in the form of lanthanide-labelled proteins.
Second, using the same synthetic principles laid out in this preprint, other chelators could be synthesised and adapted to better enhance certain properties of the CLC. For example, additional functionalities could be introduced into the molecule to modulate the wavelengths at which absorption or fluorescence occur.
Third, both the chelator and the identity of the central ion may be further customised by modifying their binding affinity. Polydentate chelators may also be designed to replace selected ligands on the central ion to form interesting new complexes with chiral properties, which could be especially useful in the characterisation of biomolecules.
In summary, the interdisciplinary nature and significance of the findings presented in this preprint are particularly promising. I look forward to the day that they click into place.
Questions for authors
- Some proteins, such as metalloenzymes, also contain a central metal ion. Would this pose potential challenges to the use of these ligands or lanthanide ions? Was this also a consideration in your lanthanide selection process?
Posted on: 11 March 2019 , updated on: 29 September 2019
doi: https://doi.org/10.1242/prelights.9300
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Structural basis of respiratory complexes adaptation to cold temperatures
Lens Placode Modulates Extracellular Matrix Formation During Early Eye Development
Generalized Biomolecular Modeling and Design with RoseTTAFold All-Atom
Also in the pharmacology and toxicology category:
Pervasive sublethal effects of agrochemicals as contributing factors to insect decline
Mixed Alkyl/Aryl Phosphonates Identify Metabolic Serine Hydrolases as Antimalarial Targets
Optical Control of G-Actin with a Photoswitchable Latrunculin
Also in the synthetic biology category:
Discovery and Validation of Context-Dependent Synthetic Mammalian Promoters
Genetically encoded multimeric tags for intracellular protein localisation in cryo-EM
Dissecting aneuploidy phenotypes by constructing Sc2.0 chromosome VII and SCRaMbLEing synthetic disomic yeast
preLists in the biochemistry category:
Preprint Peer Review – Biochemistry Course at UFRJ, Brazil
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biochemistry deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the pharmacology and toxicology category:
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
Drug use in special populations
Any drugs that are being used in special populations: Patients with liver and kidney failure, in paediatrics, in geriatrics, and in pregnant or lactating patients. Includes the discovery of factors that could potentially affect drug use in these special populations.
| List by | Zhang-He Goh |
Toxicology of toxicants, existing therapeutics, and investigational drugs
Preprints that describe the toxicology of environmental pollutants and existing and upcoming drugs. Includes both toxicokinetics and toxicodynamics, as well as technological improvements that will help in the characterisation of this field.
| List by | Zhang-He Goh |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Advances in Drug Delivery
Advances in formulation technology or targeted delivery methods that describe or develop the distribution of small molecules or large macromolecules to specific parts of the body.
| List by | Zhang-He Goh |
Also in the synthetic biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)