Defining totipotency using criteria of increasing stringency
Preprint posted on 3 March 2020 https://www.biorxiv.org/content/10.1101/2020.03.02.972893v1
Capturing totipotency in the dish: gold standards for analyzing embryonic stem cell contributions combined with molecular analyses.
Selected by Teresa RayonCategories: developmental biology
Summary:
Developmental biology is the study of how a single cell (the zygote) generates the multitude of cell types that exist in an adult organism. The initial divisions that the mammalian embryo undertakes over the first days of development increase the number of cells and retain the ability to generate a full organism. Subsequently, at the blastocyst stage, the embryo will go on to generate the first tissue types in the embryo: the epiblast (Epi), the extraembryonic (ExE) trophectoderm (TE), and primitive endoderm (PrE). Totipotency is the ability to generate a whole organism and the extraembryonic structures required during embryo development from a single cell. Over the past years, numerous reports have been published characterizing embryonic stem cells that retain totipotency features, expanding their potential compared to regular embryonic stem cells (ESCs) that contribute to the embryo proper. However, the ability to capture totipotency in vitro hasn’t been fully achieved yet.
In this preprint, Posfai, Schell and Janiszewski re-assess the totipotency features of two recently published embryonic stem cells with expanded potential (L-EPSCs and D-EPSCs). The authors compare the transcriptome and gene regulatory networks of L-EPSCs and D-EPSCs and early pre- and post- implantation mouse embryos at a single cell level to identify the correspondence between in vitro ESCs and their in vivo counterparts. Unexpectedly for cells shown to be totipotent, EPSCs were found to be transcriptionally closer to the pre- and post-implantation epiblast. Next, the authors test the ability of ESCs to transdifferentiate into trophectoderm stem cells (TSCs) and show that no embryonic stem cell passes this test. Finally, they perform the ultimate assay to assess their developmental potential: the ability of ESCs to contribute to embryonic and extraembryonic structures in vivo. They do so by generating chimeras and analysing embryos at E4.5, E6.5 and in E12.5 placentas. The tested embryonic stem cells with expanded potential (L-EPSCs and D-EPSCs) can be found in the outer layer of the blastocyst at E4.5, but none express CDX2, the specific marker of the TE lineage. From the ESCs with expanded potential, only D-EPSCs show a small contribution to extraembryonic structures of the post-implantation embryo (Figure 1). Altogether, the analyses on this preprint indicate that EPSCs capacity to generate extraembryonic tissues is very limited, and that their transcriptional signature does not correspond to totipotent blastomeres, such as those from morulae.
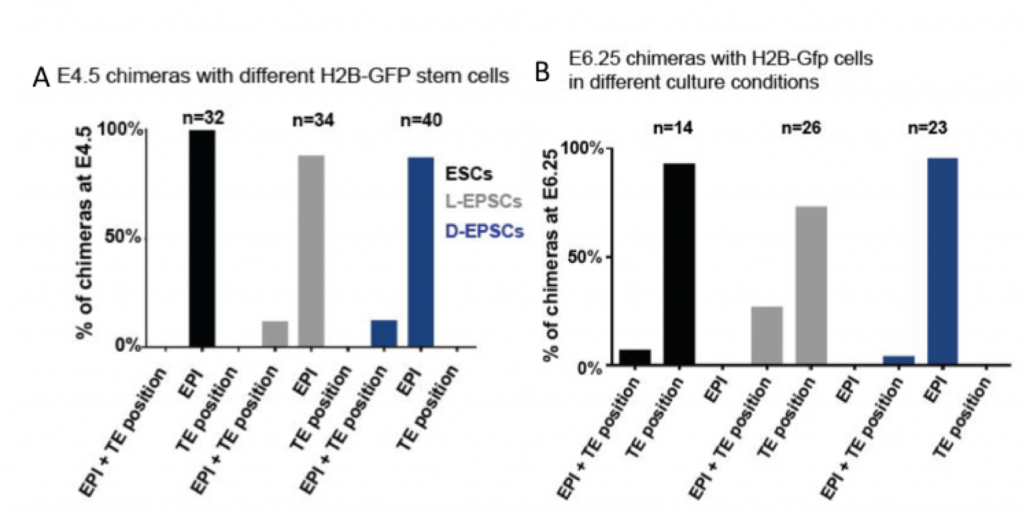
Why I think this work moves the field forward
There are now robust blastoid, 2D- and 3D- gastruloid, and all sort of organoid protocols to generate structures from stem cells in vitro that recapitulate certain aspects of embryo development. These protocols combined with the capacity to culture totipotent ESCs that can give rise to the entire conceptus would allow us to recapitulate embryo development without the need for sperm and an egg, and might enable more efficient generation of chimeric animals for research and organ production for transplantation.
With this in mind, it is crucial that totipotency is defined by accurate criteria. I really like the effort of the labs in this preprint to compile and perform rigorous comparisons for reproducibility and standardization of totipotency features. Unlike most reports, this work benchmarks several stem cells for their expanded potential, allowing the direct comparison of all of them in a single manuscript. In particular, the deep transcriptional characterization of the cells, and the assessment of their in vivo contributions with marker genes, challenges the totipotent potential of expanded stem cells. The selected criteria can be used to summarize other reports in the literature where totipotency features were described (see table below).
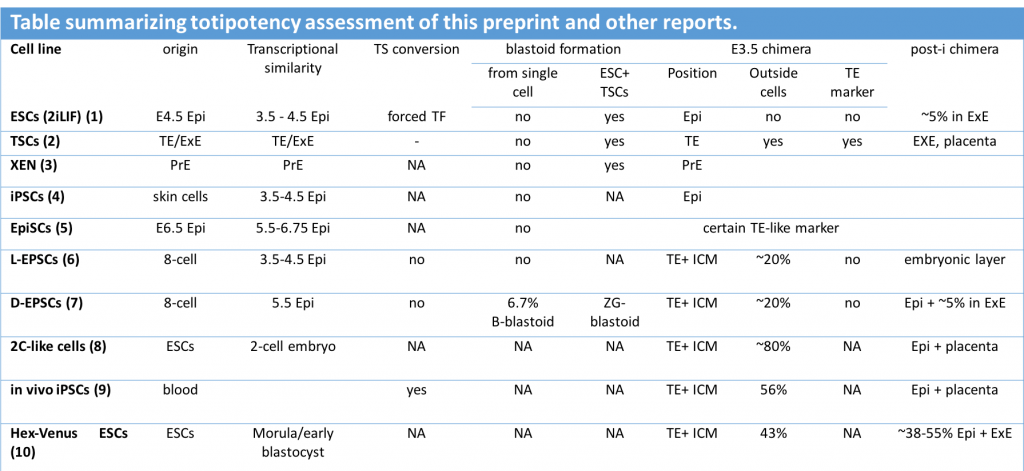
This preprint sets the baseline for future analyses on totipotency and might be useful when revisiting previous literature where totipotency was captured in vitro.
Questions to authors
Q1. How stringent do the authors think that teratoma assays are? What is the authors’ opinion on cells having expanded potential if the stem cells generate trophoblast-derived cells in teratomas assays shown by marker expression?
Q2. The authors show that a reduced percentage of D-EPSCs can contribute to the TE/ExE ectodem in blastoids, and analysis of the transcriptional signature of blastoids is closer to E4.5 blastocysts. Do the authors think that blastoids may be closer to hatching embryos, and this is why the TE signature resembles ExE ectoderm? If that is the case, do the authors think that generating blastoids that are equivalent to the expanding blastocyst (~E3.5) might highlight an expanded in vitro potential of D-EPSCs?
Q3. Why do the authors think that cells with expanded potential can be found in outside positions more often than standard 2i/LIF ESCs but are unable to express the TE marker Cdx2?
References
- Q.-L. Ying, J. Wray, J. Nichols, L. Batlle-Morera, B. Doble, J. Woodgett, P. Cohen, A. Smith, The ground state of embryonic stem cell self-renewal. Nature. 453, 519–23 (2008).
- S. Tanaka, T. Kunath, A. K. Hadjantonakis, A. Nagy, J. Rossant, Promotion of trophoblast stem cell proliferation by FGF4. Science (New York, N.Y.). 282, 2072–5 (1998).
- T. Kunath, D. Arnaud, G. D. Uy, I. Okamoto, C. Chureau, Y. Yamanaka, E. Heard, R. L. Gardner, P. Avner, J. Rossant, Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development (Cambridge, England). 132, 1649–61 (2005).
- K. Takahashi, S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126, 663–76 (2006).
- P. J. Tesar, J. G. Chenoweth, F. A. Brook, T. J. Davies, E. P. Evans, D. L. Mack, R. L. Gardner, R. D. G. McKay, New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 448, 196–9 (2007).
- J. Yang, D. J. Ryan, W. Wang, J. C.-H. Tsang, G. Lan, H. Masaki, X. Gao, L. Antunes, Y. Yu, Z. Zhu, J. Wang, A. A. Kolodziejczyk, L. S. Campos, C. Wang, F. Yang, Z. Zhong, B. Fu, M. A. Eckersley-Maslin, M. Woods, Y. Tanaka, X. Chen, A. C. Wilkinson, J. Bussell, J. White, R. Ramirez-Solis, W. Reik, B. Göttgens, S. A. Teichmann, P. P. L. Tam, H. Nakauchi, X. Zou, L. Lu, P. Liu, Establishment of mouse expanded potential stem cells. Nature. 550, 393–397 (2017).
- Y. Yang, B. Liu, J. Xu, J. Wang, J. Wu, C. Shi, Y. Xu, J. Dong, C. Wang, W. Lai, J. Zhu, L. Xiong, D. Zhu, X. Li, W. Yang, T. Yamauchi, A. Sugawara, Z. Li, F. Sun, X. Li, C. Li, A. He, Y. Du, T. Wang, C. Zhao, H. Li, X. Chi, H. Zhang, Y. Liu, C. Li, S. Duo, M. Yin, H. Shen, J. C. I. Belmonte, H. Deng, Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell. 169, 243-257.e25 (2017).
- T. S. Macfarlan, W. D. Gifford, S. Driscoll, K. Lettieri, H. M. Rowe, D. Bonanomi, A. Firth, O. Singer, D. Trono, S. L. Pfaff, Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature. 487, 57–63 (2012).
- M. Abad, L. Mosteiro, C. Pantoja, M. Cañamero, T. Rayon, I. Ors, O. Graña, D. Megías, O. Domínguez, D. Martínez, M. Manzanares, S. Ortega, M. Serrano, Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 502, 340–5 (2013).
- S. M. Morgani, M. A. Canham, J. Nichols, A. A. Sharov, R. P. Migueles, M. S. H. Ko, J. M. Brickman, Totipotent embryonic stem cells arise in ground-state culture conditions. Cell reports. 3, 1945–57 (2013).
Posted on: 16 March 2020
doi: https://doi.org/10.1242/prelights.17565
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the developmental biology category:
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Temporal constraints on enhancer usage shape the regulation of limb gene transcription
OGT prevents DNA demethylation and suppresses the expression of transposable elements in heterochromatin by restraining TET activity genome-wide
preLists in the developmental biology category:
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (2 votes)
(2 votes)