FUS gene is dual-coding with both proteins united in FUS-mediated toxicity
Preprint posted on 14 April 2020 https://www.biorxiv.org/content/10.1101/848580v2
Categories: cell biology, molecular biology, neuroscience
Background:
The term proteome defines the set of proteins known to be expressed from a fixed set of genes, typically the genome of an organism. The concept of ‘one gene one polypeptide’ has been long revised to include our understanding of processes such as alternative splicing and ribosomal frameshifting, the latter best known as a strategy employed by viruses to increase proteomic complexity from a limited set of genes [1]. Additional modes of non-conventional gene expression in both prokaryotes and eukaryotes, with the potential to vastly increase proteomic complexity are now increasingly appreciated. Thus conventional genome annotations relying on criteria like a single open reading frame (ORF) per transcript and a minimum codon length, are now challenged by the concept of alternative ORFs. These alternative ORFs can give rise to functional protein products by a variety of mechanisms including cap-independent translation [2], translation from RNAs previously designated as non-coding [3], or additional translation upstream or downstream of the annotated ORFs. The use of alternative start codons is a strategy common to many examples of alternative ORFs [4].
In this exciting new preprint, Brunet et al. report the identification of an alternative ORF in the FUS coding sequence which encodes a protein they call altFUS, and show that it may at least be partially to blame for FUS-associated toxicity. FUS is a nuclear-resident RNA-binding protein whose variants demonstrate altered cytoplasmic localization and aggregation, characteristically linked to diseases such as amyotrophic lateral sclerosis (ALS), and rare forms of frontotemporal dementia (FTD) [5].
Key Findings:
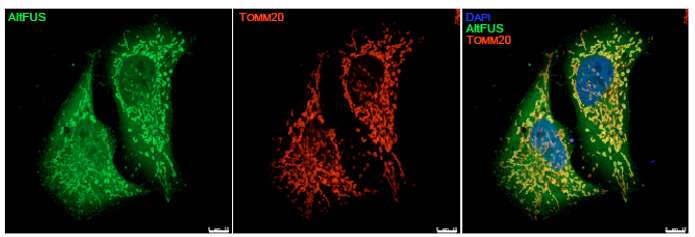
Utilizing the OpenProt resource they had previously developed [6], the authors explored the coding potential of the FUS gene to identify a predicted frame shifted ORF, resulting in a 169 amino acid protein. The specific transcript for this predicted coding protein, altFUS, was found to be highly abundant in brain tissue. Mining of existing ribosome profiling data revealed accumulation of initiating ribosomes at the altFUS initiating methionine, indicating actual translation of this alternative ORF. Several unique altFUS peptides were identified from proteomic datasets, allowing the generation of a custom antibody to detect altFUS. This enabled the specific detection of altFUS expression, which was remarkably observed not just in cell lines but pathological tissue samples and iPSC-derived motor neurons. Further, altFUS was found to be a mitochondria-localized protein (Figure 1), capable of altering the mitochondrial network, and acting cooperatively with FUS in reducing mitochondrial membrane potential. Based on insights from protein-protein interaction pathways, the authors investigated the contribution of altFUS to cellular stress and autophagy. Interestingly, they found that altFUS promotes assembly of the characteristic cytoplasmic aggregates seen in disease-linked FUS mutants; and that it alone can inhibit autophagy in the absence of FUS.
Mutations in FUS that were previously believed to be synonymous and potentially harmless, were found to instead affect the altFUS coding sequence, leading to enhanced TDP-43 aggregation, another pathological hallmark of ALS. Finally, using an in vivo Drosophila model of neurodegeneration, the authors show that while altFUS alone could not recapitulate the disease phenotype, both FUS and altFUS expression are required for the toxicity reflected by loss of motor neuron activity.
Why I picked this preprint:
This work by Brunet et al. is a good example of how our progress with understanding disease mechanisms could be limited by the existing knowledge of players involved. In this study, altFUS is found to be not just endogenously expressed but also at least partially responsible for the alterations in physiology, previously attributed to FUS. Further, mutations previously believed to be synonymous in the context of FUS, take on new meaning in case of altFUS. The contribution of alternative gene expression to disease pathology is already an expanding field, particularly in our understanding of various neurodegenerative conditions. The best known example of this is Huntington’s Disease where toxic polypeptides of variable lengths arise from the expansion of trinucleotide repeats (encoding poly-glutamine stretches) on the HTT (Huntingtin) gene. Mounting evidence for additional non-conventional modes of translation opens up the possibility of a complement of unannotated proteins with potentially unknown physiological functions: a new ‘dark proteome’ [7].
References for further reading:
- Programmed Ribosomal Frameshifting Goes Beyond Viruses. (2006) doi: 10.1128/microbe.1.521.1
- Cap-Independent Translation: What’s in a Name? (2018) https://doi.org/10.1016/j.tibs.2018.04.011
- Translation and functional roles of circular RNAs in human cancer. (2020) https://doi.org/10.1186/s12943-020-1135-7
- Leaderless Transcripts and Small Proteins Are Common Features of the Mycobacterial Translational Landscape. (2015) https://doi.org/10.1371/journal.pgen.1005641
- TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. (2010) https://doi.org/10.1016/S1474-4422(10)70195-2
- OpenProt: a more comprehensive guide to explore eukaryotic coding potential and proteomes. (2019) https://doi.org/10.1093/nar/gky936
- Alternative ORFs and small ORFs: shedding light on the dark proteome. (2019) https://doi.org/10.1093/nar/gkz734
Questions for the authors:
- What fraction of known disease-linked proteins do you think possess alternative ORFs? Do specific sequence features e.g. intrinsically disordered regions represent a higher likelihood of leading to alternative ORFs?
- altFUS is expressed even under normal conditions and in control tissues, but could specific conditions alter the stoichiometry of expression of the two proteins? If both are necessary for cooperative toxicity, then could altered stoichiometry be a natural mechanism to counteract toxicity under normal conditions?
- Does altFUS possess a canonical mitochondrial targeting sequence or does it associate with mitochondria only under certain conditions (e.g. α-synuclein associates with mitochondria under conditions of stress)? This could help to understand whether altFUS has normal physiological functions at the mitochondria regardless of its pathological contributions.
Posted on: 12 May 2020 , updated on: 13 May 2020
doi: https://doi.org/10.1242/prelights.20492
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Also in the molecular biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Nanos2+ cells give rise to germline and somatic lineages in the sea anemone Nematostella vectensis
Plant plasmodesmata bridges form through ER-driven incomplete cytokinesis
AND
Plasmodesmata act as unconventional membrane contact sites regulating inter-cellular molecular exchange in plants
Also in the neuroscience category:
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Synergistic olfactory processing for social plasticity in desert locusts
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the molecular biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)