Intercellular telomere transfer extends T cell lifespan
Preprint posted on 9 October 2020 https://www.biorxiv.org/content/10.1101/2020.10.09.331918v1
Categories: cell biology, immunology
Background
Mammalian telomeres are TTAGGG repeats that mark chromosome ends. At each cell division, telomeres shorten due to the uncomplete replication of DNA ends. When telomere length shortens to a critical point, cells stop proliferating and enter a state known as replicative senescence (Campisi and d’Adda di Fagagna, 2007). Stem and cancer cells can circumvent this problem by upregulating telomerase, a ribonucleoprotein able to elongate telomeres by synthesizing new telomeric repeats. Some cancer cells adopt an alternative lengthening of telomeres (ALT) mechanism based on homologous-recombination (Doksani, 2019). While telomerase-dependent and -indepent phatways have been extensively studied, the possibility that telomeres are transferred between cells to counteract telomere shortening had never been explored—until now. In their preprint, Lanna and colleagues suggest that T lymphocytes, key cells in the immune system, elongate their telomeres by acquiring those of antigen presenting cells (APC).
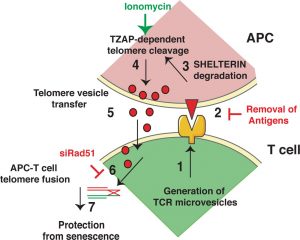
From Vaz et al, 2020 (CC-BY-NC-ND 4.0 International license).
Key observation: At the immunological synapse, T cell telomeres elongate while APC telomeres shorten.
The authors mixed human primary T cells with autologous APCs and specific antigens to obtain immunological synapses. Then they measured telomere length by either in situ hybridisation (IF-FISH) in fixed cells (still involved in the synapses) or by telomere restriction fragment (TRF) or qPCR analyses in the two different cell populations (sorted based on their cellular markers). They observed that synapse formation was followed by telomere elongation in T cells to the expense of APC telomeres.
The mechanism was still unclear; however, it seemed to differ from the ‘canonical’ ones. Telomerase-dependent elongation mechanisms were excluded by reproducing the results in TERT (the catalytic subunit of telomerase) knockout T cells. Moreover, from the observation that telomeres still elongate in T cells upon immunological synapse formation, despite chemical inhibition of DNA synthesis (by 48h treatment with aphidicolin or thymidine), the authors excluded ALT-based elongation mechanisms.
The proposed model, step by step:
- Upon formation of the immunological synapse, APCs downregulate the telomere protection machinery, upregulate TZAP-mediated telomere trimming and release telomere vesicles.
The authors observed that activated APCs release telomeric DNA into the extracellular space. After labelling APC DNA with the thymidine analogue BrdU, the presence of extracellular DNA was tested by BrdU IP in cell-free supernatant. Not only was DNA present in the extracellular space, but this was telomeric DNA, as revealed by dot-blot using telomeric probes. Moreover, DNase degraded the extracellular telomeric DNA only upon pre-treatment with detergents, thus suggesting that telomeric DNA is contained in lipidic vesicles. This was further confirmed by transmission electron microscopy (TEM) and immunogold-based telomeric detection.
Activated APCs downregulated the telomere protection machinery (shelterin complex) and upregulated the telomeric zinc-finger associated protein TZAP, which promotes trimming of telomeric DNA ends (Li, 2017). Consistent with TZAP binding to telomeres only in the presence of low shelterin levels (Li, 2017), overexpression of shelterin reduced telomere vesicle release. Conversely, depletion of shelterin components induced the release of telomere-containing vesicles. TZAP was contained in the telomeric vesicles and required for their formation.
- APC telomeric DNA is integrated into T cell chromosome ends
To monitor the presence of APC telomeric DNA at T cell chromosome ends, the authors incubated T cells with vesicles containing BrdU-labelled APC telomeres. From the analysis of T cells metaphase spreads the authors concluded that 10% of the telomeric signal, detected by IF-FISH, is also BrdU positive, thus suggesting co-localization with APC DNA. A similar result was obtained with APCs live-labelled with fluorescent telomeric probes. From the observation that T cell chromosomes with APC-derived telomeres are destroyed upon incubation with T7 endonuclease (an enzyme that cleaves DNA mismatches and non-β DNA structures including recombination intermediates), the authors concluded that APC telomeres are integrated within T cell chromosomes.
- The recombination protein RAD51 associates with APC telomeres and facilitates integration of APC telomeric repeats at T cell chromosome ends.
The authors observed that telomere vesicles contained several DNA damage proteins, including the recombination protein RAD51. Activated APCs depleted for RAD51 formed telomere vesicles with the same efficiency as control cells, as monitored by flow cytometry. However, those telomers had shorter single-stranded overhangs compared to controls. They co-localized with the T cell telomeres to a lower extent and failed to promote T cell telomere elongation, as monitored by quantitative (Q)-FISH.
- APC telomeres support T cell expansion in vitro and in vivo.
Upon activation, T cells expanded exponentially for ~10 days and then reached a plateau phase, corresponding to an increase in senescence markers, as monitored by beta-galactosidase staining. Incubating proliferating T cells with telomere vesicles (both autologous and allogenic from mismatched human and mouse APCs) reduced the percentage of senescent cells and sustained T cell expansion, thus suggesting that APCs support T cell proliferation in vitro.
To test whether the same process occurs in mice, the authors exploited the OT-II OVA system: they introduced into mice live-stained T cells expressing a transgenic T cell receptor for the chicken ovalbumin (obtained from the OT-II mice) and ovalbumin-pulsed APCs with fluorescently labelled telomeres. One day later, they observed that antigen-specific T cells had acquired fluorescent telomeres from APCs. Additionally, they demonstrated that administration of APC telomere vesicles sustains the expansion of a subset of T cells upon vaccination of the mice with ovalbumin, thus recapitulating the proposed model in vivo.
Why I chose this preprint
I was extremely curious about this preprint since few days after it was posted my Twitter was full of notifications citing it–and it is not even exactly my field! I love the novelty and the provoking model suggested and the possible implications this finding could have, in particular in ageing-related diseases and cancer immunotherapy.
Questions for the author
The authors prove that resting T cells are still able to integrate APC telomeres. Does it imply that T cells not involved in the same immunological synapse, and possibly other cell types, could uptake those vesicles as well? Do T cells control this process somehow? More philosophically, it would be interesting to know whether other cell types could undergo the same telomere exchange.
I am curious about the mechanism of telomeric integration. It would be interesting to monitor DNA damage induction at telomeres in both the cell types and eventually monitor the composition of the telomere overhangs contained in the telomere vesicles. The authors propose that RAD51 role is to protect the ssDNA overhangs, which are important for the ‘fusions’ of APC with T cell recipient telomeres. Is that the only role for RAD51? Does a recombination intermediate form (as suggested by the T7 endonuclease assay)? Does the homologous recombination machinery in the recipient T cells have any role in the process? Could this imply a possible use of DNA repair inhibitors in modulation of autoimmunity?
At last, could senescent T cells be “reactivated” by up-taking APC telomeres?
References:
- Campisi, J., d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol(2007).
- Doksani, Y. The Response to DNA Damage at Telomeric Repeats and Its Consequences for Telomere Function. Genes (Basel) (2019).
- Li, J. S. Z. et al. TZAP: a telomere-associated protein involved in telomere length control. Science (2017)
Posted on: 23 October 2020
doi: https://doi.org/10.1242/prelights.25432
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Also in the immunology category:
Spatial transcriptomics elucidates medulla niche supporting germinal center response in myasthenia gravis thymoma
Prenatal inflammation reprograms hyperactive ILC2s that promote allergic lung inflammation and airway dysfunction
NAD+ metabolism is a key modulator of bacterial respiratory epithelial infections
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the immunology category:
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (3 votes)
(3 votes)