MCM complexes are barriers that restrict cohesin-mediated loop extrusion
Preprint posted on 15 October 2020 https://www.biorxiv.org/content/10.1101/2020.10.15.340356v1
Article now published in Nature at http://dx.doi.org/10.1038/s41586-022-04730-0
Categories: cell biology
Background:
The eukaryotic genome is folded in a highly organised and complex way within the nucleus; how it is folded can influence several processes including gene expression. Using chromatin conformation capture (Hi-C), chromosomes were shown to be organised into topological associated domains (TADs) reflecting DNA regions which prefer to interact with each other (intradomain) (1). These TADs are thought to form through the actions of a macromolecular holocomplex called the structural maintenance of chromosomes (SMC) cohesin complex (SMC1-SMC3-SCC1; 2,3) and its interactions with the CCCTC-binding factor (CTCF), which is located at TAD boundaries. The movement of SMC along the chromatin pulls DNA into a ‘loop’ which stops growing when the complex dissociates or reaches convergent CTCF sites at boundary regions; this model is currently known as ‘loop extrusion’ and has been shown to regulate gene expression by allowing distant genes to interact with each other within TADs (4,5). However, the genomic landscape however is not sparse, with numerous factors occupying and interacting with the chromatin, all of which could prevent loop extrusion machinery movement. How cells may deal with these possible conflicts is largely unknown nor is how chromatin occupying factors may influence the structure of the genome. One such macromolecular complex is the mini-chromosome maintenance (MCM) complex. MCM is loaded onto DNA, facilitated by the loading protein Cdt1 (6), at origins of replication by the origin recognition complex (ORC) during late mitosis and G1 phase, becoming activated during S-phase to drive DNA replication. Here, the authors ask if MCM complexes can alter DNA ‘loop’ extrusion and affect genome structure in germline and somatic cells.
Key Findings:
The authors investigated genome architecture during the transition between oocytes to zygotes (OZT) in cells deficient for the loading of MCM onto the DNA. During this transition, ooctyes are fertilised to become a one-cell zygote. ‘MCM loss’ zygotes were generated by preventing the factor geminin from being degraded as the presence of geminin stops Cdt1 directed loading of MCM during G1 phase (6).
- Loss of MCM impedes loop formation and TAD boundary establishment
Using single nucleus Hi-C (snHi-C; 7), a modified form of Hi-C, the authors examined loop formation and TAD boundaries in chromatin extracted from maternal and paternal pronuclei from control and ‘MCM loss’ cells. By looking at peaks and TADs associated with previously known co-ordinates of CTCF-bound regions (i.e loop forming regions), the authors show that MCM loss results in increased peaks at these co-ordinates correlating with increased intermediate and longer-range interactions being detected suggesting loss of MCM affects CTCF-bound regions and TAD boundaries. They confirm this affect is dependent upon the activities of cohesin.
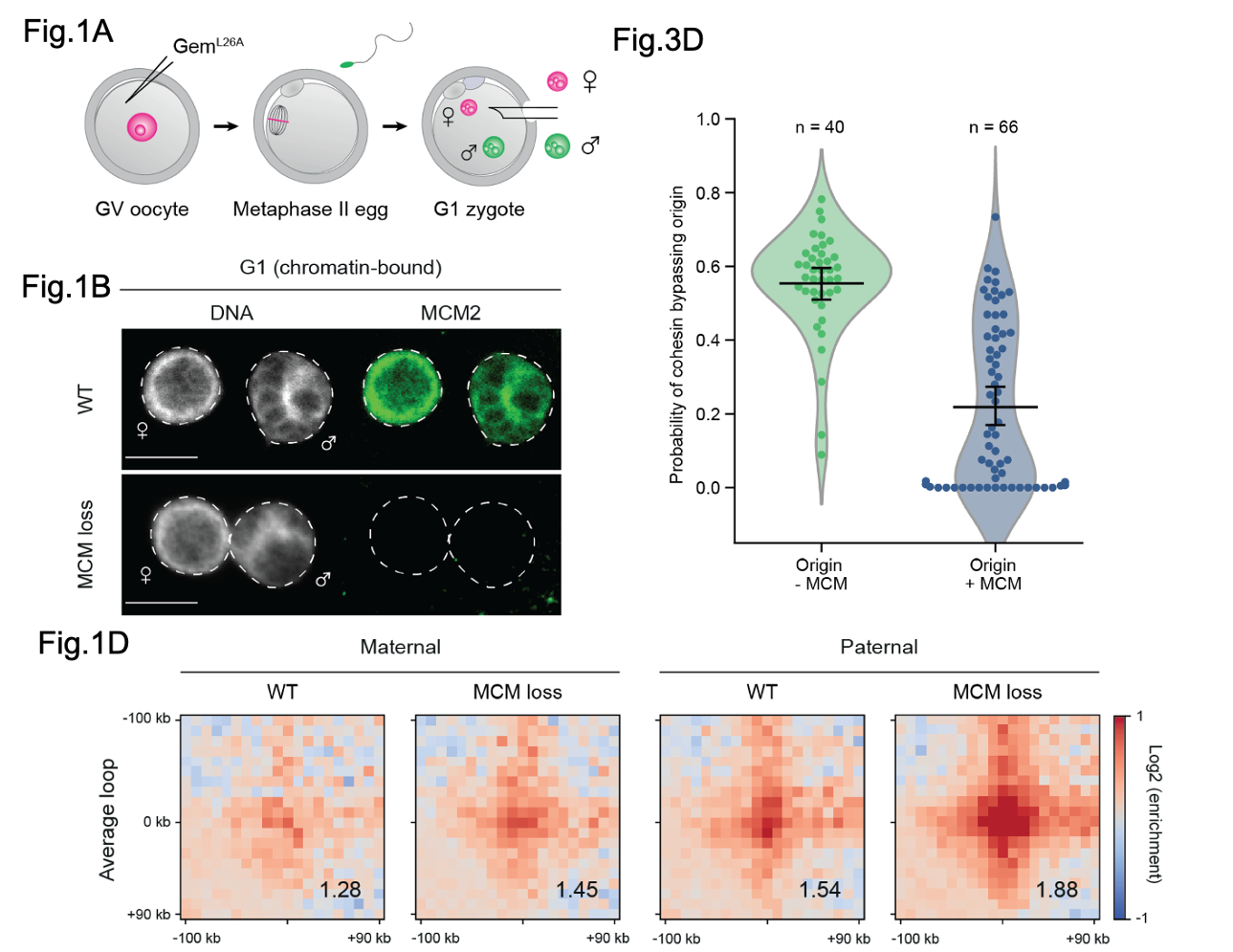
Figure shows selected data from Dequeker et al. Fig1A. shows a schematic diagram of the approach the authors used to prevent MCM loading onto the chromatin. Fig1B. shows representative images of MCM localisation in MCM positive and MCM negative nuclei. Fig1D. shows the average loops and TADs for control and MCM loss nuclei. Fig3D. Graph shows the probability of cohesin moving past MCM at an origin in control and MCM loss cells. Figures extracted from Dequeker et al. 2020 and modified under a CC-BY-NC 4.o international license.
- Loss of MCM affects CTCF-bound peaks but MCM does not establish TAD boundaries
Loss of MCM was not associated with changes to the size of DNA loops but their analysis suggests it does alter CTCF-bound regions. Therefore, the authors reasoned that if MCM affected CTCF directed loop formation, the increased peak strength from MCM loss cells around these regions could be due to MCM affecting how CTCF establishes these regions and how the TAD boundaries are insulated from one and other. By deleting CTCF in ‘MCM loss’ cells, the insulation (i.e the separation between one boundary and the next) between TAD boundaries was weaker than in CTCF depleted control cells suggesting MCM can act as a barrier to the formation of these regions however, MCM did not affect TAD boundary establishment, which is entirely lost in CTCF- depleted cells and not re-gained when MCM is also lost. These effects are unlikely due to MCM being involved in the release of cohesin from the chromatin and instead, supports the actions of MCM as a random barrier to loop extrusion.
- Loss of MCM affects loop extrusion and TAD boundaries similarly in germline and somatic cells
To ask if the effect of MCM loss on loops and TADs could occur in somatic (non-germline) cells, the authors degraded MCM using an inducible degradation system in HCT116 cells (human colon cancer cells) and collected cells after synchronisation in G1 phase and following MCM degradation. Using bulk Hi-C, they show similar peak patterns to zygote cells, albeit more subtle. Though more work is needed to investigate this, transcriptional changes in these MCM degraded cells suggest that the barrier like properties of MCM could affect gene expression.
- MCM complexes can physically prevent movement of cohesin
Next, the authors directly investigated if MCM could interact with cohesin on the chromatin. Here, the authors use total internal reflection fluorescence microscopy (TIRFM), which is a high-resolution microscopy technique in which a thin layer of the sample is excited on the surface only exciting surface fluorophores. This means other (deeper) fluorophores are not excited which improves background noise. Here, the authors perform an in vitro assay in which DNA containing an origin region was spread on a slide and purified licensing factors added to the DNA to allow MCM to become loaded at the origin. After these were washed off, they flow cohesin over this DNA strand and track its movements revealing cohesin can pass MCM bound regions, but it was 3x less frequent, with some failing to traverse entirely. They also noted that both single and (predominantly) double hexamers of MCM could interfere with cohesin movement and supporting MCM as random barrier, which can be overcome in some cases. Using quantitative modelling, they support their in vitro and in vivo findings additionally revealing that barrier location could also influence extruded DNA loops, i.e if barriers are far away from each other or can be passed, then the size of the DNA loop is likely to be unaffected, perhaps explaining why MCM loss does not affect DNA loop size.
What I liked about this preprint:
I enjoyed reading this preprint very much. The authors took an approach they previously developed and applied it to reveal novel biology surrounding a core complex involved in DNA replication and the extrusion of DNA, whilst utilising additional techniques, cell systems and modelling to support their findings. I found the use of TIRFM very interesting to look at protein complex interactions on DNA. Often, the DNA can be thought of as a static, linear molecule and this paper is a reminder that the DNA landscape is in fact a complex environment of interacting factors which can influence genome architecture, and if perhaps altered, could affect gene expression leading to diseases states.
Questions for Authors:
Q1: Ordinarily geminin is degraded at the metaphase/anaphase boundary but here you are retaining it during meiosis II and in G1 cell cycle stage cells. Does the retention of geminin in your cells have any wider consequences on their development?
Q2: Why do you think MCM loss shows a greater effect on paternal chromatin when compared to maternal chromatin?
Q3: You show that MCM barriers could potentially affect gene expression and, as you mention, MCM loading defects have been linked to diseased states in humans. From these transcriptional changes, do you observe dysregulation of any factors that could correlate with defective MCM loading in the context of pathology?
Q4: Your model suggests that cohesin could overcome an MCM barrier. Could you speculate on what you think might happen to allow cohesin to overcome this obstacle? For example, there is evidence to suggest condensin complexes can bypass one and other by forming a DNA structure known as a Z-Loop.
References:
- Szabo, Q., Bantignies, F., and Cavalli, G. Principles of genome folding into topologically associated domains. (2019) Science Advances.
- Gibcus, J.H., Samejima, et al. A pathway for mitotic chromosome formation. (2018) Science
- Rao, S.S., Huang, S., et al. Cohesin loss eliminates all loop domains. (2017) Cell
- Fudenberg, G., Abdennur, N., Imakaev, M., Goloborodko, A. and Mirny, L. Emerging evidence of chromosome folding by loop extrusion. (2017) Cold Spring Harb. Symp. Quant. Biol.
- Davidson, I., Bauer, B., Goetz, D., Tang, W., Wutz, G., and Peters, J. DNA loop extrusion by human cohesin. (2019) Science.
- Ticau, S., Friedman, L., Ivica, N., Gelles, J., and Bell, S. Single-molecules studies of origin licensing reveal mechanisms ensuring bidirectional helicase loading. (2015) Cell.
- Flyamer, I., Gassler, J., Imakaev, M. et al. Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. (2017) Nature.
Posted on: 19 November 2020
doi: https://doi.org/10.1242/prelights.25735
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)