Microfluidic protein isolation and sample preparation for high resolution cryo-EM
Preprint posted on 21 February 2019 https://www.biorxiv.org/content/10.1101/556068v1
Article now published in Proceedings of the National Academy of Sciences at http://dx.doi.org/10.1073/pnas.1907214116
Purification and cryoEM structure determination of an untagged protein complex from a single microliter of cell lysate
Selected by David WrightCategories: biochemistry, biophysics, molecular biology
Background
Cryogenic electron microscopy (cryoEM), with recent improvements in hardware and software, has become a very powerful technique for the high resolution structure determination of proteins (1). There are many advantages of CryoEM over crystallography; however one of the most important is that typically CryoEM structures can be determined from a few micrograms of purified material, rather than the milligram quantities usually needed for crystallography. In this preprint the authors take this one step further and, using an ingenious combination of techniques, are able to solve the structure of an untagged protein from as little as one microliter of cell lysate.
Results
Figure 1 very clearly shows a basic protocol for this work, which I will briefly describe. Commercial antibodies specific to the α4 subunit of the 20S proteasome were treated with a kit to produce Fab fragments: these are minimal regions of antibodies containing only a single antigen binding site, rather than the two found on a complete antibody. Fab fragments were used rather than intact antibodies to prevent any potential cross-linking and aggregation. These Fabs were then biotinylated via a photocleavable linker and bound to paramagnetic streptavidin beads: the beads were then incubated with the lysate from 1 microliter of commercial HeLa cells. The proteasome complexes in the lysate bound to the Fabs, which were in turn bound to the streptavidin beads, immobilised in the magnetic trap and washed to remove all other protein and non-protein contaminants. Elution of the highly pure complexes was achieved by irradiation at a specific UV wavelength to detach the Fab fragments from their biotin tags. This high concentration sample was then applied to EM grids using the Cryowriter technique and frozen without blotting (2).
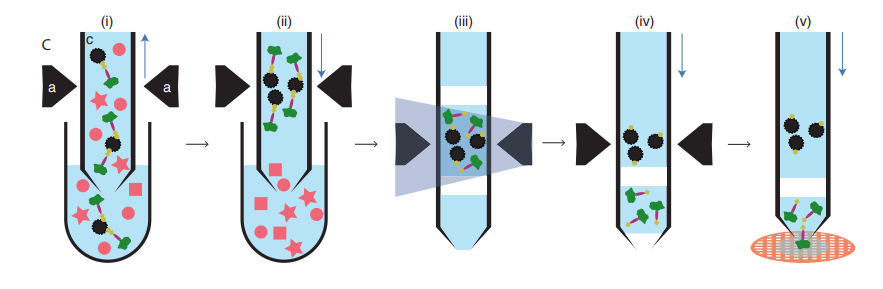
Figure 1. Schematic work-flow for microfluidic protein isolation and cryo-EM grid preparation – (i) The lysate is incubated with resin-immobilised Fabs (ii) A magnetic field is applied to separate the Fab-bound protease particles (iii) A UV pulse breaks link between biotin and Fab (iv) Fab-bound protease particles are released (v) The eluted samples are applied to EM grids
The structure obtained from the data (figure 3) has an estimated resolution of 3.5 Å, which is similar to the previous cryoEM structure (3), suggesting that the technique does not affect the resolution significantly. The authors report that the structure largely agrees with previous crystallographic data. Interestingly, subunits β4-6 are less well resolved in the EM map, which the authors attribute to their flexibility. Additionally, the Fab fragments can be seen weakly in the density, which may have been useful for assigning the orientation of these pseudo-symmetric particles in the reconstruction.
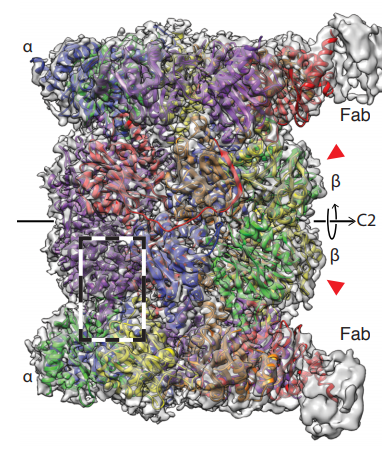
Figure 3. 3D reconstruction of the human 20S proteasome – Final EM map of the 20S proteasome coloured by subunit, showing the C2 symmetry axis and Fab fragments in grey only.
As an another control, the authors add Tobacco Mosaic Virus at a known concentration in the wash buffer, which resulted in a 1.9 Å structure from the same micrographs as used for the proteasome. This again suggests that the set up does not prevent the generation of high resolution structural data.
This article may have a large impact on the structural biology field. It shows that it is possible to solve structures of protein complexes expressed at native levels from very small volumes of cells. This work could pave the way for the examination and quantification of native protein complexes.
Comments and questions
- Since this is a native sample, it might be expected that, in addition to the 20S proteasome, there would be other super-complexes such as the 26S proteasome. Did the authors see any evidence of this subpopulation?
- How long was the data collection? Was there any obvious preferential orientation of the proteasomes?
- How well does the proteasome express? Would this work for less well expressed proteins?
- Might this setup be useable with other expression tags, which could be used in combination with overexpression to promote the formation of certain complexes?
Why I chose this article
The main reason I chose the article is because of how novel the technology is. As I have previously worked on crystallography, I think it’s amazing that structures can be solved from such small volumes of cells. I’m really looking forward to seeing this technology being used to probe the structure and stoichiometry of native protein complexes.
References
- Kühlbrandt W. The Resolution Revolution. Science. 2014;343(6178):1443.
- Arnold SA, Albiez S, Opara N, Chami M, Schmidli C, Bieri A, et al. Total Sample Conditioning and Preparation of Nanoliter Volumes for Electron Microscopy. ACS Nano. 2016 2016/05/24;10(5):4981-8.
- da Fonseca PCA, Morris EP. Cryo-EM reveals the conformation of a substrate analogue in the human 20S proteasome core. Nature Communications. 2015 07/02/online;6:7573.
Posted on: 6 March 2019
doi: https://doi.org/10.1242/prelights.9204
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the biochemistry category:
Structural basis of respiratory complexes adaptation to cold temperatures
Lens Placode Modulates Extracellular Matrix Formation During Early Eye Development
Generalized Biomolecular Modeling and Design with RoseTTAFold All-Atom
Also in the biophysics category:
Structural basis of respiratory complexes adaptation to cold temperatures
Actin polymerization drives lumen formation in a human epiblast model
Learning a conserved mechanism for early neuroectoderm morphogenesis
Also in the molecular biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Nanos2+ cells give rise to germline and somatic lineages in the sea anemone Nematostella vectensis
Plant plasmodesmata bridges form through ER-driven incomplete cytokinesis
AND
Plasmodesmata act as unconventional membrane contact sites regulating inter-cellular molecular exchange in plants
preLists in the biochemistry category:
Preprint Peer Review – Biochemistry Course at UFRJ, Brazil
Communication of scientific knowledge has changed dramatically in recent decades and the public perception of scientific discoveries depends on the peer review process of articles published in scientific journals. Preprints are key vehicles for the dissemination of scientific discoveries, but they are still not properly recognized by the scientific community since peer review is very limited. On the other hand, peer review is very heterogeneous and a fundamental aspect to improve it is to train young scientists on how to think critically and how to evaluate scientific knowledge in a professional way. Thus, this course aims to: i) train students on how to perform peer review of scientific manuscripts in a professional manner; ii) develop students' critical thinking; iii) contribute to the appreciation of preprints as important vehicles for the dissemination of scientific knowledge without restrictions; iv) contribute to the development of students' curricula, as their opinions will be published and indexed on the preLights platform. The evaluations will be based on qualitative analyses of the oral presentations of preprints in the field of biochemistry deposited in the bioRxiv server, of the critical reports written by the students, as well as of the participation of the students during the preprints discussions.
| List by | Marcus Oliveira |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the biophysics category:
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
66th Biophysical Society Annual Meeting, 2022
Preprints presented at the 66th BPS Annual Meeting, Feb 19 - 23, 2022 (The below list is not exhaustive and the preprints are listed in no particular order.)
| List by | Soni Mohapatra |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Biophysical Society Meeting 2020
Some preprints presented at the Biophysical Society Meeting 2020 in San Diego, USA.
| List by | Tessa Sinnige |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Biomolecular NMR
Preprints related to the application and development of biomolecular NMR spectroscopy
| List by | Reid Alderson |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
Also in the molecular biology category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (No Ratings Yet)
(No Ratings Yet)