Nix induced mitochondrial fission, mitophagy, and myocyte insulin resistance are abrogated by PKA phosphorylation
Preprint posted on 4 November 2019 https://www.biorxiv.org/content/10.1101/825828v1.full
Article now published in Autophagy at http://dx.doi.org/10.1080/15548627.2020.1821548
Nix's function beyond mitophagy: role of a lipotoxicity-induced Nix in myocyte insulin resistance.
Selected by Sandra Franco IborraCategories: cell biology
This work establishes a connection between lipotoxicity, mitophagy activation and reduced insulin sensitivity in skeletal muscle cells. Lipotoxicity is a stress insult caused by the accumulation of lipid intermediates resulting in insulin resistance. The muscle tissue is central to this concept since most of the postprandial glucose uptake occurs in the muscle (Shulman et al., 1990). Indeed rodents fed a high-fat diet accumulate diacylglycerols, ceramides and triglycerides in the muscle tissue, leading to the activation of signaling pathways (Yu et al., 2002; Itani et al., 2002) that have been shown to inhibit the insulin receptor substrate-1 (IRS1) (Li et al., 2014). Altered muscle mitochondrial function is intimately linked to obesity and insulin resistance (Lowell & Shulman 2005; Szendroedi et al., 2011). Mitophagy ensures the quality of the mitochondrial pool by selectively eliminating those dysfunctional mitochondria. Dysfunctional mitochondria are specifically recognized by receptors that connect the mitochondria with LC3-II in the autophagosomal membrane. Some receptors can be mitochondrial proteins (Nix, FUNDC1, PHB) while others are non-mitochondrial proteins that recognize ubiquitinated chains on the mitochondrial surface (Martinez-Vicente, 2017). Excessive removal of dysfunctional mitochondria by mitophagy in the muscle has been suggested to contribute to muscle insulin resistance (Fu et al., 2018).
Previous work performed by the authors showed increased expression of the mitophagy receptor Nix upon lipotoxicity (Mughal et al., 2015). In this manuscript, the authors explore the mechanisms of lipotoxicity-mediated Nix activation and undercover new roles for Nix beyond mitophagy induction.
Key findings
High-fat diet led to increases in lipid species in rat soleus muscle including diacylglycerols, ceramides, triglycerides, phosphatidic acid and alterations in cardiolipin composition. Autophagy-related genes (such as Beclin-1, ATG3, -5, -12) were moderately decreased, together with PGC-1α and some mitochondrial enzymes. Previous work has already shown that high-fat diet induces the expression of Nix, which is a known mitophagy receptor (Mughal et al., 2015).
Overexpression of Nix in C2C12 myoblasts led to increased mitochondrial fragmentation, presumably due to calcium-mediated dephosphorylation of DRP1, increased mitophagy and altered insulin-stimulated glucose uptake. Lipotoxicity induction using palmitate treatment recapitulated those phenotypes and also stimulated increased Nix expression. Importantly, Nix knockdown abrogated mitochondrial fragmentation, increased mitophagy and altered glucose uptake. Therefore, in addition to its role as a mitophagy receptor, Nix is an important regulator of lipotoxicity-induced mitochondrial dysfunction, mitophagy and insulin resistance.
The next step was to investigate which signaling pathway could be regulating Nix function. It turns out that Nix contains a conserved PKA consensus motif that can be phosphorylated at Serine-212. Treatment of C2C12 myoblasts with pharmacological PKA activators led to increased phospho-Nix (pNix) and prevention of Nix-induced mitochondrial depolarization, suggesting that Ser-212 could represent an inhibitory phosphorylation site. To confirm this hypothesis, C2C12 cells were treated with palmitate and pharmacological PKA activators. PKA activation in palmitate-treated cells led to the rescue of mitochondrial depolarization, mitochondrial fragmentation and mitophagy induction and activation of insulin-stimulated glucose uptake. Thus, Ser-212 is a PKA phosphorylation site that modulates Nix-induced mitophagy and insulin sensitivity.
Moreover, cell fractionation studies indicate that pNix is exclusively localized to the cytosolic fraction. Interestingly, PKA-responsive Ser-212 is localized to the conserved interacting domain of the molecular chaperone family 14-3-3. Could this family of chaperones be responsible for Nix translocation from ER and/or mitochondria to the cytosol? Indeed, Nix co-immunoprecipitates with 14-3-3β chaperone and this interaction is enhanced upon treatment with pharmacological PKA activators. Moreover, co-expression of Nix and 14-3-3β counteracts Nix effects on mitochondrial function, mitophagy and insulin sensitivity.
One interesting observation is the relationship between Nix activation and insulin resistance. Nix overexpression in C2C12 myotubes leads to IRS1 phosphorylation and inhibition. IRS1 Ser-1101 phosphorylation has been shown to be mediated by PKC Ө and/or p70S6K. At the same time, phosphatidic acids are direct activators of the mTOR-p70S6K pathway. Interestingly, Nix overexpression or palmitate treatment in C2C12 myoblasts increased p70S6K phosphorylation in a Nix-dependent manner. On the other hand, phospholipase 6 knockdown prevents p70S6K expression, presumably due to the lack of phospholipase 6 activity that leads to decreased phosphatidic acid levels. These observations point at a link between excessive mitochondrial turnover and impaired insulin sensitivity.
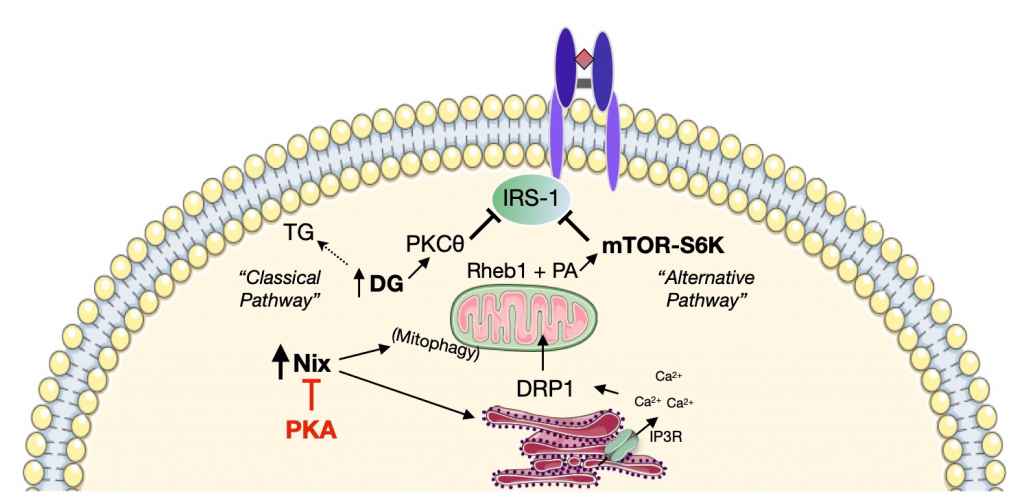
Lipotoxicity-induced Nix overexpression leads to DRP1-mediated mitochondrial fragmentation, increased mitochondrial turnover and desensitization of insulin receptor signaling. PKA activation inhibits Nix function and restores insulin signaling.
Overall, this manuscript shares light on the signaling cascade triggered by lipotoxicity in the muscle and suggests that PKA pharmacological activation might promote insulin responsiveness in the myocytes.
Why I like this preprint
The mitophagy field has experienced exponential growth in the past decade. However, most of the work is focused on non-mitochondrial receptors and PINK1/parkin mitophagy. Nix was originally described to play a role in mitophagy taking place during reticulocyte maturation, when mitochondria have to be eliminated from the erythrocyte (Schweers et al 2007; Sandoval et al 2008). However, mitophagy is also a mechanism that allows the regulation of mitochondrial content in response to changing metabolic conditions. This manuscript describes a link between increased lipid content, activation of a mitophagy receptor and increased mitochondrial turnover. Moreover, the results shown here increase our understanding of Nix function and regulation beyond mitophagy.
Questions for the authors
- The metabolomic studies performed in rats fed with high-fat diet show alterations in cardiolipin composition. Cardiolipin is a critical inner mitochondrial membrane lipid involved in mitochondrial cristae morphology and stability. Do you think that changes in cardiolipin composition can lead to mitochondria dysfunction? Which would be the relationship between alterations in cardiolipin composition and Nix function?
- Nix is a protein that contains a transmembrane domain and that has been described in the outer mitochondrial membrane. How would you explain that mitochondrial-targeted Nix constructs don’t stimulate mitophagy, while the ER/SR targeted ones do?
- How do you reconcile the fact that there is a moderate downregulation in autophagy-related genes upon lipotoxic stress, while mitophagy is being stimulated?
References
- Fu T, Xu Z, Liu L, Guo Q, Wu H, Liang X, Zhou D, Xiao L, Liu L, Liu Y, et al. Mitophagy Directs Muscle-Adipose Crosstalk to Alleviate Dietary Obesity. Cell Rep 2018; 23:1357–72.
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002; 51:2005–11.
- Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, Littman DR, Birnbaum MJ, Polakiewicz RD. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem 2004; 279:45304–7.
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 2005; 307:384-7.
- Martinez-Vicente M. Neuronal mitophagy in neurodegenerative diseases. Front Mol Neurosci 2017;10:64.
- Mughal W, Nguyen L, Pustylnik S, da Silva Rosa SC, Piotrowski S, Chapman D, Du M, Alli NS, Grigull J, Halayko AJ, et al. A conserved MADS-box phosphorylation motif regulates differentiation and mitochondrial function in skeletal, cardiac, and smooth muscle cells. Cell Death Dis 2015; 6:e1944.
- Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 1990; 322:223–8.
- Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2011; 8:92-103.
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 2002; 277:50230–6.
Posted on: 13 December 2019
doi: https://doi.org/10.1242/prelights.15760
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Fetal brain response to maternal inflammation requires microglia
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |











 (No Ratings Yet)
(No Ratings Yet)