Persistence of quantal synaptic vesicle recycling following dynamin depletion
Preprint posted on 12 June 2020 https://www.biorxiv.org/content/10.1101/2020.06.12.147975v1
Categories: neuroscience, physiology
Background and Approach:
During chemical neurotransmission, synaptic vesicles fuse with the presynaptic plasma membrane and release neurotransmitters into the cleft, but the membrane belonging to the vesicles must also be retrieved to reform vesicles. While mechanisms of endocytosis of synaptic vesicles are somewhat contentious, it is generally accepted that the GTPase dynamin plays an essential role in the process by forming rings that pinch off and recover endocytic vesicles from the plasma membrane. Because the triple KO mouse of all three dynamin isoforms is not viable, and dynamin inhibitors have been found to be nonspecific (1), it has been difficult to fully assess the role of dynamin in synaptic vesicle endocytosis.
To directly address this question, the authors used hippocampal neuronal cultures from mice with constitutive and conditional loss of dynamin isoforms. One of the key methods the authors use to examine vesicle release and endocytosis is the pH-sensitive fluorescent reporter vGluT1-pHluorin (2). This reporter is quenched when vesicles are intact due to their acidic interior and becomes fluorescent upon fusion with the plasma membrane due to the neutral extracellular environment. This allows Afuwape et al. to determine the time course of fusion and vesicle recovery following fusion. They examine dynamin’s function in neurotransmission and synaptic vesicle recycling during both strong stimulation and during single synaptic vesicle endocytosis.
Key Findings:
While synapses lacking all three dynamin isoforms (Dnm TKO) showed no deficits in morphology, these synapses exhibited a decrease in the number of both total and docked synaptic vesicles, compared to synapses lacking only dynamin 3. Accordingly, Dnm TKO neurons, but not Dnm 2 KOs, demonstrated impaired excitatory neurotransmission: evoked excitatory postsynaptic currents were decreased and synapses demonstrated facilitation to trains of stimuli, a common indicator of decreased evoked release probability.
Though the expectation might be to find arrested synaptic vesicle recycling following high frequency stimulation, the authors find that different synapses respond distinctly to the absence of all dynamins. Only 40% of boutons showed a deficit in endocytosis, the rest either showing no, or only partial deficit compared to boutons lacking only dynamin 3.
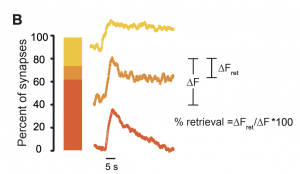
This significant result implies that dynamin-independent endocytosis of vesicles occurs at central synapses. It also suggests that dynamin-dependent and independent endocytosis may have unequal importance across synapses. Even more strikingly, single synaptic vesicle fusion events show a similar distribution of vGlut1-pHluorin amplitudes dwell times in the absence of just dynamin 3 or all three dynamins, suggesting that dynamins are not required for single vesicle endocytosis.
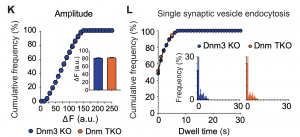
To provide further support for the idea that dynamins are only required for multi-vesicular endocytosis, the authors more closely examined the fraction of events which decayed rapidly (<1s), with a short dwell time (1-10s), or with a longer dwell time (>10s). For synapses that demonstrated slowed endocytosis after high frequency stimulation, a very small fraction had long dwell times compared to all synapses from TKO or control neurons, supporting the idea that dynamin is most critical for multi-vesicle endocytosis.
These results imply that there must be other proteins which can carry out single vesicle recycling in addition to dynamin. The authors pharmacologically tested the involvement of actin, DRP-1 and Arp2/3 complex, which have previously been implicated in synaptic vesicle endocytosis (3, 4), finding that they do not play a major role in the recycling of spontaneously fused synaptic vesicles.
Perspective:
Though other studies have suggested dynamin independent vesicle endocytosis (5, 6), this study elegantly shows that single synaptic vesicles can be recycled in the absence of dynamin. Determining the proteins responsible for scission during endocytosis of synaptic vesicles in its absence is an exciting new direction for the field. This work also points to the possibility that different synapses operate with distinct endocytosis mechanisms, both within the population of excitatory synapses and between excitatory and inhibitory synapses, which is a potential mechanism for physiological diversity.
Questions for authors and open questions for future study:
- Under basal conditions, are the three dynamin isoforms likely to function at each synapse or does their distribution vary across synapses? What is the current understanding of the degree to which they are unique or redundant in hippocampal synapses?
- The authors find that depleting all three dynamins reduces release probability in both excitatory and inhibitory synapses, mentioning reduced vesicle availability and limited viable release sites as two potential mechanisms. How might one distinguish between these two possibilities? Is it also possible that dynamins are involved more directly in exocytosis?
- The authors justify the use of dynamin 3 KOs as controls by stating that this provides the most consistent comparisons with littermates and because previous studies showed no significant functional or structural deficits. I’m wondering if dynamin 1 and dynamin 2 are upregulated at the mRNA or protein level in dynamin 3 KO neurons and what effect this might have over developmental time.
- Is it possible that some of the rapid decay events correspond to kiss-and-run type release events in which the fusion pore opens and closes without fully fusing with the plasma membrane?
- If observed events all correspond to full fusion of vesicles in which all neurotransmitter is released, are individual events with different dwell times likely to be different in terms of the physiological effect on the post-synapse?
References:
- Park, R.J., Shen, H., Liu, L., Liu, X., Ferguson, S.M., and De Camilli, P. (2013). Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. Journal of cell science 126, 5305-5312.
- Voglmaier, S.M., Kam, K., Yang, H., Fortin, D.L., Hua, Z., Nicoll, R.A., and Edwards, R.H. (2006). Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron 51, 71-84.
- Lee, E., and De Camilli, P. (2002). Dynamin at actin tails. Proc Natl Acad Sci U S A, 161-166
- Orth, J.D., Krueger, E.W., Cao, H., and McNiven, M.A. (2002). The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc Natl Acad Sci U S A 99, 167- 594 172.
- Xu, J., McNeil, B., Wu, W., Nees, D., Bai, L., and Wu, L.G. (2008). GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci 11, 45-53.
- J. Van Hook, W.B. Thoreson Rapid synaptic vesicle endocytosis in cone photoreceptors of salamander retina J. Neurosci., 32 (2012), pp. 18112-18123
Posted on: 30 June 2020 , updated on: 14 July 2020
doi: https://doi.org/10.1242/prelights.22478
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Synergistic olfactory processing for social plasticity in desert locusts
Also in the physiology category:
Unlocking the secrets of kangaroo locomotor energetics: Postural adaptations underpin increased tendon stress in hopping kangaroos
Changes in surface temperatures reveal the thermal challenge associated with catastrophic moult in captive Gentoo penguins
Torpor energetics are related to the interaction between body mass and climate in bats of the family Vespertilionidae
preLists in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Also in the physiology category:
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)