Segmentation of the Zebrafish Brain Vasculature from Light Sheet Fluorescence Microscopy Datasets
Preprint posted on 22 July 2020 https://www.biorxiv.org/content/10.1101/2020.07.21.213843v1
Article now published in Current Protocols at http://dx.doi.org/10.1002/cpz1.443
Categories: cell biology, developmental biology
Background
Zebrafish are useful models to study vascular biology, due to factors including their high genomic similarity to humans, rapid ex utero development, fecundity, larval transparency, and the availability of multiple reporter lines for multiple sub-cellular structures. Vascular diseases are a leading cause of death worldwide and many factors underlying pathology are currently not fully understood. Imaging modalities such as light sheet fluorescence microscopy (LSFM) allow the acquisition of vascular information in great anatomical detail throughout extended periods of time.
Current challenges in quantifying the zebrafish cranial vasculature include a lack of robust segmentation and quantification approaches. This is because most vascular research in zebrafish has been performed in the trunk vasculature; moreover, the cerebral vasculature is topologically challenging to study. LSFM is a relatively novel technique with great potential for the study of the vasculature in significant detail. However, approaches to validate image analysis methods are broadly lacking. Given that there is no gold standard for vascular segmentation, in their work, Kugler et al aim to overcome limitations in the knowledge about segmentation quality, by performing detailed validation of the segmentation methods using simulated data, manual measurements, and various in vivo biological datasets (1).
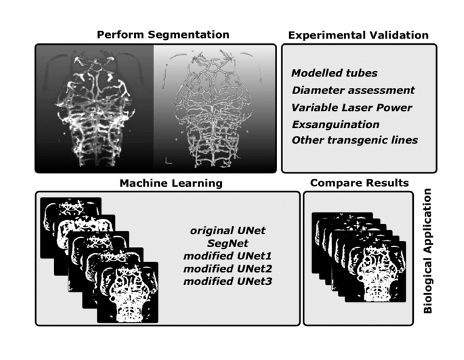
Key findings and developments
The work begins by exploring vessel enhancement using a shape-based filter with the assumption of local vascular tubularity. The Sato Enhancement filter (SE) already implemented into Fiji was evaluated for its applicability to images of the zebrafish vasculature acquired with LSFM. SE delivered meaningful results if data is presented as bright-on-dark. Applying SE to the simulated hollow tubes converted these to filled tubes when enhancement scale was approximately at the size of tubes. SE was able to convert double-to-single peak intensity distributions if applied at the scale of tubes, suggesting that lumenized and unlumenized vessels would both be enhanced similarly. However, there was a tendency for the simulated tube width to appear broader after enhancement, so any subsequent segmentation would need to be tuned to recover the correct vessel width.
To evaluate segmentation accuracy, vessel diameters obtained after applying the proposed enhancement and segmentation methodologies were compared to those obtained by the ‘gold standard’ manual measurement. When comparing full-width-half-maximum (FWHM) with the manual diameter measurements, no statistically significant differences were found. The authors conclude that the FWHM can be used to measure vascular diameter in zebrafish reporter lines, although outliers need to be considered. Upon comparing FWHM and manual measurements to general filtering and SE after thresholding, no significant differences were found. No segmentation method introduced significant artificial bias.
Segmentation robustness was assessed by processing data with varying signal properties. Two approaches were used for evaluation, namely data acquisition with a controlled decrease in image quality, and data augmentation by artificial noise addition. CNR was successfully decreased with this approach. Both were followed by segmentation after using general filtering methods (GF) and SE. The authors conclude that both segmentation approaches were robust over a broad range of contrast-to-noise ratio (CNR) levels
Segmentation sensitivity was assessed by using datasets with a predictable vascular volume difference via image acquisition before and after exanguination. No statistical difference was found in cerebral vascular volume after GF between control and exanguinated samples. SE was more sensitive, allowing extraction of true biological differences. In summary, evaluation of all segmentation approaches indicated that SE-based segmentation is more successful than the GF-based approach.
The authors then went on to quantify cerebral vascular volume throughout zebrafish development between days 3 and 5 post fertilization, using the transgenic reporter line Tg(kdrl:HRAS-mCherry)s916. The data suggested that SE-processed data were less variable and therefore lower sample numbers would be required to extract biologically relevant vascular volumes. The authors then evaluated whether this approach could be generalizable to other transgenic lines. They found that segmentation in reporter lines under the fli1a promoter displayed, in addition to vascular information, enhancement and segmentation of non-vascular signal. Quantification of the cerebral vascular volume showed a significantly higher vascular volume under the fli1a promoter in all examined reporter lines. Altogether, the authors conclude that vascular volume can be extracted in other transgenic lines, but accuracy and precision are reduced. They suggest that future work might include pre-processing to enhance vascular and decrease non-vascular signal, exclusion of non-connected components, exclusion of objects under a specified size-threshold, or exclusion based on peripheral position.
The authors then aimed to use deep learning approaches for segmentation of zebrafish vascular data from the single transgenic Tg(kdrl:HRAS-mCherry)s916. For this, they trained an original U-Net, SegNet, and three modified U-Nets (dU-Net1-3) on the training dataset using segmented masks obtained after SE as the ground truth. The resulting trained network was then applied to the evaluation dataset. U-Net architectures seemed to deliver better results than SegNet, which over-segmented the vasculature. The original U-Net and dU-Net1 delivered the best results. There was a tendency for the deep learning methods to systematically over-estimate the vascular volume. The deep learning approaches used in this study result in typical run times of between 7 and 23 minutes for segmenting a full stack. This was less than half the time required using SE-based segmentation, suggesting a time benefit for using deep learning methods.
What I like about this preprint
The authors systematically tested and compared segmentation methods for studying the zebrafish vasculature. I find this is a useful study and an important step forward for a current hindrance which is data analysis. I imagine advances like this will be a positive feedback loop for tools such as LSFM.
References
1. Kugler EC, et al Segmentation of the zebrafish brain vasculature from light sheet fluorescence microscopy datasets, bioRxiv, 2020.
Posted on: 3 September 2020
doi: https://doi.org/10.1242/prelights.24422
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Also in the developmental biology category:
Temporal constraints on enhancer usage shape the regulation of limb gene transcription
OGT prevents DNA demethylation and suppresses the expression of transposable elements in heterochromatin by restraining TET activity genome-wide
Actin polymerization drives lumen formation in a human epiblast model
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the developmental biology category:
GfE/ DSDB meeting 2024
This preList highlights the preprints discussed at the 2024 joint German and Dutch developmental biology societies meeting that took place in March 2024 in Osnabrück, Germany.
| List by | Joyce Yu |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Society for Developmental Biology 79th Annual Meeting
Preprints at SDB 2020
| List by | Irepan Salvador-Martinez, Martin Estermann |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EDBC Alicante 2019
Preprints presented at the European Developmental Biology Congress (EDBC) in Alicante, October 23-26 2019.
| List by | Sergio Menchero et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)