Skeletal muscle mitochondrial dysfunction in mice is linked to bone loss via the bone marrow immune microenvironment
Preprint posted on 9 December 2020 https://www.biorxiv.org/content/10.1101/2020.12.09.417147v1
Happy muscle, happy bone! The mechanism that links muscle dysfunction, bone marrow inflammation and bone loss.
Selected by Andrea IrazokiCategories: cell biology, physiology
BACKGROUND
Mitochondrial dysfunction has been extensively associated with loss of muscle bulk and atrophy [1]. Progressive loss of muscle function is, in turn, a critical factor for the development of bone alterations, such as osteoporosis, due to the reduction of bone strength caused by a decrease in the mechanical loading on the skeleton [2]. During bone remodelling carried out by bone cells (osteoclasts and osteoblasts), bone marrow (BM) immune cells undertake specific functions that regulate the function of bone cells. In fact, in the context of autoinflammatory diseases, BM T cells provide a specific pro-inflammatory environment that allows osteoclast differentiation, which results in bone resorption. Thus, bearing in mind that during muscle atrophy, pro-inflammatory cytokines can be secreted to the system, the authors hypothesized that there could be a mechanism linking muscle mitochondrial dysfunction, bone marrow signalling and bone loss.
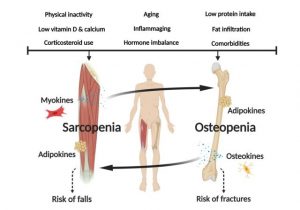
Figure 1: Crosstalk between skeletal muscle and bone. Inflammatory cytokines from the muscle (myokines) can signal to the bone and trigger bone loss (osteopenia). On the other hand, cytokines released by bone cells (osteokines) can also signal to the skeletal muscle and promote muscle wasting (sarcopenia). Edited from Kirk B., et al., 2020.
In order to address this hypothesis, the authors used a CRIF1 knockout (KO) mouse model for skeletal muscle mitochondrial dysfunction. CRIF1 is a component of the large subunit of the mitoribosome, which has an essential role in the translation of mitochondrial oxidative phosphorylation (OxPhos) polypeptides. Indeed, alterations in the OxPhos system have been widely related to decreased mitochondrial respiratory capacity, and therefore, mitochondrial dysfunction [3]. Thus, using skeletal muscle-specific CRIF1 KO (MKO) mice, the authors collect extensive transcriptomic data as well as data on the cell and tissue biology of BM and muscle that, along with functional assays, allows them to properly describe the impact of muscle mitochondrial dysfunction on the inflammatory response and bone remodelling.
KEY FINDINGS
First, the authors validated the phenotype observed in the MKO mice: OxPhos dysfunction and stress response in the skeletal muscle. This is characterized by decreased expression levels of the OxPhos complex subunits, accumulation of abnormal, swollen mitochondria with disrupted cristae, and increased expression levels of proteins related to stress responses, such as the unfolded protein response (UPR) or ER stress. Besides, evaluation of the physical performance of MKO mice showed loss of body mass, motor coordination and strength, suggesting that muscle mitochondrial dysfunction results in muscle dysfunction and atrophy. Furthermore, the authors describe bone loss in these mice, characterized by alterations in various bone histomorphometric parameters including: bone mass, mineral density, trabecular bone number, thickness and volume, osteoblast and osteoclast activity, and serum levels of the bone formation and reabsorption markers (procollagen I N-terminal propeptide and C-terminal telopeptide of collagen, respectively).
In order to gain insights into the connection between muscle mitochondrial dysfunction and bone loss, the authors performed transcriptomic analyses in the EDL muscle, in which significantly higher levels of the myokine FGF21 were found in MKO mice compared to wild-type (WT). Nevertheless, downregulation of FGF21 in MKO mice did not rescue or restore bone loss, suggesting that the effects observed in the bones of these mice were independent of the role of FGF21.
Considering that muscle mitochondrial dysfunction has been described to trigger the upregulation of inflammatory cytokines [4], the authors performed various analyses in BM to test whether increased inflammation in the BM caused by muscle dysfunction could lead to bone loss. The following were assessed: i) expression levels of pro-inflammatory molecules in BM cells of MKO and WT by transcriptomic analyses, ii) presence of immune cells in the BM and their characterization by flow cytometry, and iii) serum levels of proinflammatory cytokines. The results of these analyses suggested that MKO mice present local (not systemic) BM inflammation and T cell infiltration, which could be driving bone loss. Besides, gene enrichment analyses revealed that genes associated with myopathy, osteoporosis, activation of the immune system and adipogenesis were upregulated in BM cells. Particularly, the authors observed activation of the CXCL12-CXCR4 axis which, besides its implication in angiogenesis and metastasis, is involved in certain inflammatory autoimmune disorders such as rheumatoid arthritis. Indeed, treatment of MKO mice with a CXCR4 antagonist not only resulted in reduced T cell infiltration and pro-inflammatory cytokine levels in the BM, but also in the prevention of bone loss. Thus, these data suggest that mitochondrial muscle dysfunction promotes the presence of a pro-inflammatory environment in the BM, characterized by activation of the CXCL12-CXCR4 axis, which in turn results in bone loss. Last but not least, these data were validated in human subjects with hip fracture and lower body mass index, which correlated with a higher inflammatory response, therefore confirming the role of inflammation in the development of bone complications in the context of muscle dysfunction.
WHY I CHOSE THIS PREPRINT
Considering the lack of evidence regarding the molecular pathways that link muscle atrophy and bone remodelling, I found the approaches that the authors of the preprint used to shed light on this subject very interesting. They obtained valuable data on the transcriptomics of muscle and bone marrow in the context of muscle mitochondrial dysfunction, as well as extensively characterized the residing immune cells. In my opinion the authors elegantly provide new insights into the link between muscle atrophy and bone loss, which will be very relevant in the context of systemic effects of muscle diseases.
MY QUESTIONS TO THE AUTHORS
- Considering that you were able to discard the effect of FGF21 in the connection between muscle dysfunction and bone loss, do you speculate that other myokines could be acting as a bridge between muscle dysfunction and the BM? If so, do you have an idea of what molecule or molecules could be playing that role?
- If the adipogenesis could be reduced or prevented in the BM of MKO mice, would you expect a reduction of the pro-inflammatory environment, and therefore, bone loss prevention?
- Do you think that other types of mitochondrial dysfunction like alterations in mitochondrial dynamics, mitophagy, or calcium and iron homeostasis, can also result in the effects observed in the BM and bones of MKO mice?
REFERENCES
[1] Sartori R. et al., Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun., 2021. doi: 10.1038/s41467-020-20123-1.
[2] Johanssen DL et al., Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab, 2012. 97, 242-250, doi:10.1210/jc.2011-1798
[3] Boenzi S and Diodato D. Biomarkers for mitochondrial energy metabolism diseases. Essays Biochem., 2018. doi: 10.1042/EBC20170111.
[4] Missiroli S., et al., The role of mitochondria in inflammation: from cancer to neurodegenerative disorders. J Clin Med, 2020. doi: 10.3390/jcm9030740
Posted on: 17 February 2021 , updated on: 18 February 2021
doi: https://doi.org/10.1242/prelights.27438
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Fetal brain response to maternal inflammation requires microglia
Alteration of long and short-term hematopoietic stem cell ratio causes myeloid-biased hematopoiesis
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Also in the physiology category:
How the liver contributes to stomach warming in the endothermic white shark Carcharodon carcharias
Unlocking the secrets of kangaroo locomotor energetics: Postural adaptations underpin increased tendon stress in hopping kangaroos
Changes in surface temperatures reveal the thermal challenge associated with catastrophic moult in captive Gentoo penguins
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the physiology category:
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)