Sparse recurrent excitatory connectivity in the microcircuit of the adult mouse and human cortex
Preprint posted on 8 May 2018 https://www.biorxiv.org/content/early/2018/05/08/292706
Article now published in eLife at http://dx.doi.org/10.7554/elife.37349
New work from @AllenInstitute compares synaptic connectivity in human cortex to the commonly studied mouse visual cortex. Very insightful study from preproduction data -- looking forward to the full production data release!
Selected by Mahesh KarnaniCategories: neuroscience
Summary
This preprint from Tim Jarsky’s team at the Allen Institute for Brain Science shows synaptic connectivity profiles within cortical layers in neurosurgical samples of human brain and compares them thoroughly to samples from mouse brain. Compared to the commonly studied mouse visual cortex, the human frontal and temporal cortex has more synaptically connected neurons forming a denser recurrent microcircuit. The conclusions are compatible with previous work and help place findings from the mouse in a translational perspective.
Context
Understanding how the brain generates activity patterns that underlie thought and behaviour necessitates knowledge of communication protocols in local microcircuits that are composed of synaptically connected neurons. Synaptic communication in microcircuits of the rodent brain has been studied for decades, using multi-cell patch clamp recordings in acutely isolated brain slices. This work has resulted in detailed – although still incomplete – knowledge of connectivity patterns in rodent sensory cortex 1,2 and an ongoing transition toward studying how the microcircuit layout affects dynamics of brain activity and cortical computations. Similar work in the human cortex is yet to be done, although some laboratories do have access to neurosurgical samples of human brain, resected during removal of brain tumours, for example.
One such laboratory is at the Allen Institute for Brain Science, which has already contributed big datasets from many large-scale projects to the neuroscience community, creating comprehensive databases of meso-scale connectivity, marker expression, and morphologies of human and mouse neurons 3. The institute is currently working on several highly interesting projects and accelerates dissemination of results through its online database and preprinting regularly on Biorxiv. Their latest work compares connectivity in human frontal and temporal cortex to mouse visual cortex. The presented dataset is from a “pipeline’s system integration test” and is expected to lead to a comprehensive dataset, as the team starts routine operation of the workflow.
Key findings
This study is very exciting because it shows increased connectivity and synaptic weights in human cortex compared to the mouse visual cortex. The study confirms previous reports of high connectivity in human cortex 4 and gives a translational perspective compared to results obtained in mouse cortex. Whereas previous work identified complex multisynaptic events triggered by single action potentials in human cortex 4,5, this study aimed to minimize their occurrence since they can interfere with assessment of monosynaptic connectivity. Lowering the occurrence of complex events was achieved by modifying experimental variables such as decreased recording temperature and calcium and potassium concentrations of the extracellular medium compared to the previous studies.
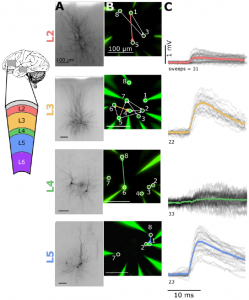
While lower than in human cortex, connectivity in the mouse visual cortex was high compared to a previous study 2. The authors have two results that bear on this and similar discrepancies in the literature. Firstly, connectivity is highly distance dependent and should thus be reported as a function of distance in order to compare across labs. Secondly, led by co-first-author Luke Campagnola, the team have devised a machine learning based method for estimating synapse detection limits during paired recordings. This approach can be used to compare results from different labs if the raw data are made available.
Why I chose this preprint
I work on connectivity in the mouse brain, and often think about how applicable these results are to our efforts to understand our own brains. Evolutionary and structural arguments suggest there is a “translational bridge”, but it is very important to have direct comparative data. The key physiological differences include the high connectivity in human cortex reported in this preprint and enlarged excitatory synapses on inhibitory interneurons 5–7. Together these endow human cortex the propensity for complex multisynaptic events triggered by single action potentials. This is exceedingly rare in rodent microcircuits 8 and could partly explain the ability of the human neocortex to perform highly complex tasks. However, it seems from this preprint that the complex events are quite sensitive to extracellular medium composition. It would be interesting to see more direct, controlled comparisons of complex events across recording conditions and species.
What next?
The authors have made a powerful pipeline for generating important results from human cortex. While it will be highly informative to use their protocols with single action potentials and firing trains in one cell at a time to characterize synaptic responses, even more could be learned through simultaneous firing protocols in multiple cells. This could reveal cooperatively scaling excitation and disynaptic inhibition 9,10 which may be drastically different in human cortex, given the differences noted so far. The presented human data are within layers and across pyramidal cells only, and it will be interesting to see in future publications how connectivity across layers and interneurons compares to mouse.
References:
- Lefort, S., Tomm, C., Floyd Sarria, J.-C. & Petersen, C. C. H. The Excitatory Neuronal Network of the C2 Barrel Column in Mouse Primary Somatosensory Cortex. Neuron 61, 301–316 (2009).
- Jiang, X. et al. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 350, aac9462-aac9462 (2015).
- http://www.brain-map.org/.
- Molnár, G. et al. Complex events initiated by individual spikes in the human cerebral cortex. PLoS Biol. 6, e222 (2008).
- Szegedi, V. et al. High-Precision Fast-Spiking Basket Cell Discharges during Complex Events in the Human Neocortex. eNeuro 4, ENEURO.0260-17.2017 (2017).
- Szegedi, V. et al. Plasticity in Single Axon Glutamatergic Connection to GABAergic Interneurons Regulates Complex Events in the Human Neocortex. PLoS Biol. 14, e2000237 (2016).
- Molnár, G. et al. Human pyramidal to interneuron synapses are mediated by multi-vesicular release and multiple docked vesicles. Elife 5, e18167 (2016).
- Brecht, M. Neuronal communication: firing spikes with spikes. Curr. Biol. 22, R633-5 (2012).
- Kapfer, C., Glickfeld, L. L., Atallah, B. V & Scanziani, M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 10, 743–53 (2007).
- Berger, T. K., Silberberg, G., Perin, R. & Markram, H. Brief Bursts Self-Inhibit and Correlate the Pyramidal Network. PLoS Biol. 8, e1000473 (2010).
Posted on: 31 May 2018
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Synergistic olfactory processing for social plasticity in desert locusts
preLists in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)