Target-specific precision of CRISPR-mediated genome editing
Preprint posted on 9 August 2018 https://www.biorxiv.org/content/early/2018/08/09/387027
Article now published in Molecular Cell at http://dx.doi.org/10.1016/j.molcel.2018.11.031
The predictability of genome editing outcome varies across target sites and primarily depends on the nucleotide in the -4 position from the PAM site. Careful selection of target site is therefore key to inducing a specific desired modification.
Selected by Rob HyndsCategories: bioinformatics, molecular biology
Background
Cas9 is an RNA-guided DNA endonuclease that operates in the CRISPR (clustered regularly interspaced short palindromic repeats) bacterial adaptive immune mechanism. In these bacteria, short lengths of DNA from plasmids or bacteriophages are transcribed to CRISPR RNAs (crRNAs) which then provide the specificity for the Cas9 endonuclease to destroy the invading pathogen. Cas9 recognition of foreign DNA relies on crRNA, which contains a 20-nucleotide recognition region known as a protospacer, and a tracrRNA which hybridises with the crRNA. The role of the crRNA-tracrRNA complex can be performed by a single guide RNA (sgRNA) and this strategy is now widely used for genome editing in mammalian cells. The Cas9-sgRNA complex binds to homologous genomic DNA where aprotospacer adjacent motif (PAM) sequence (e.g. NGG for Cas9 from S. pyogenes) is present downstream of the target sequence. Cas9 induces a double-stranded break that is then repaired by endogenous DNA repair processes and can be further manipulated by inclusion of repair templates.
When no template for repair is provided, double-stranded breaks induced by Cas9 are repaired using error-prone repair pathways. These pathways introduce frameshift insertions or deletions (indwells) that disrupt the open reading frame and generate non-functional proteins, phenocopying gene knockout. A previous paper showed that the outcome of these repair events is not random and disruption of specific target sites can have a preferred repair outcome.
A current focus of research is finding ways to improve the design of sgRNAs to successfully target the locus of interest while minimizing off-target events elsewhere in the genome. In silico prediction tools now allow researchers apply our existing knowledge of the sequence patterns that correlate with high efficiency sgRNA activity but these remain imperfect.
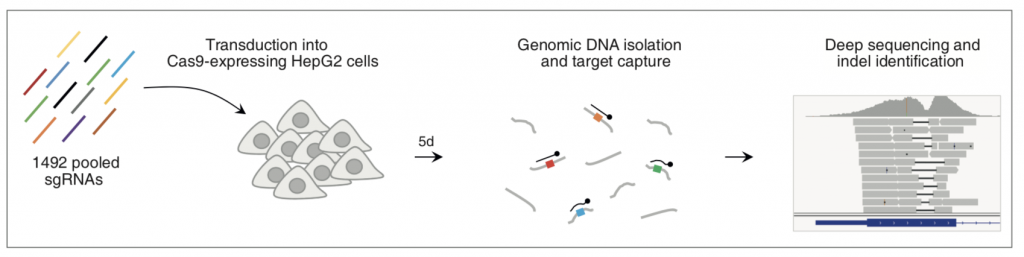
Figure 1A: Experimental Design
Key findings
In their preprint, Chakrabarti and colleagues examine the pattern of indels generated during CRISPR-Cas9-mediated gene editing in the absence of a repair template. The authors assessed repair of 1492 target sites in 450 genes in HepG2 cells using a pooled lentiviral library of sgRNAs predicted to have high activity and confirm that the outcome of editing is non-random in biological replicates. Single nucleotide (nt) indels occurred most frequently but there was a long tail in the length of indels and the preferred indel length for some target sites was as long as 56 nt. Almost 90% of indels produced frameshift but some target sites showed in-frame indel preference, suggesting that they should be avoided for gene KO studies. This, along with the observation that multiple sgRNAs seemed to have lower activity than predicted suggests that prediction algorithms can be further refined through better understanding of the activity of Cas9 in human cells. The pattern of indels at different sites varied with some having one strong preference and others having little preference between dozens of possibilities; the finding that only one-fifth of target sites (‘precise targets’) have a greater than 50% probability of inducing one specific indel is significant as predicting the specific outcome of genome editing for the remaining target sites is not easy.
Further characterisation of precise targets revealed that editing at these was more efficient. Precise targets were more likely to be insertions and more likely to be a single nucleotide in length while imprecise targets favoured deletions. Microhomology around the indel appeared to be a feature of deletions, consistent with repair by the MMEJ pathway. Some single nucleotide insertions also showed a preference for a common base suggesting that the nucleotide choice is not random. The preferred base was homologous to nucleotide -4 from the PAM sequence, which is usually one nucleotide upstream of the cleavage site. Moreover, precise targets showed base preference at the -4 position: when the target has an “A” or a “T” in the -4 position, repair is likely to result in a highly recurrent insertion but when it is a “G”, deletions are more likely and repair is less predictable.
These data clarify the role of DNA sequence in repair precision after Cas9 cleavage but the failure of algorithms based solely on sequence to predict indel profiles accurately suggests that other factors might influence the indel profiles of target sites. In this regard, the preprint finds a role for chromatin structure. Addition of a HDAC inhibitor or an EZH2 inhibitor altered the indel profiles observed for the same target sites by increasing and decreasing indel formation, respectively. The extent of these changes was similar to when DNA repair pathways are pharmacologically manipulated supporting the importance of chromatin structure. Some changes in the relative frequency of specific indels at targets sites were observed but were broadly similar to the untreated conditions. That said, the authors demonstrate that for some target sites, altering the chromatin state can change the most frequent indel and favour some indels over others. Future work should address the hypothesis that there is more complex interplay between chromatin state and DNA repair pathway choice than is currently appreciated.
Conclusions
- DNA sequence features affect the indel profiles generated after CRISPR-Cas9 gene editing.
- Chromatin structure also affects indel formation and inducing histone acetylation improves the efficiency of editing.
- Establishing and targeting precise sites will maximize the likelihood of desirable outcomes in experimental and clinical applications of gene editing.
Further Reading
Taheri-Ghahfarokhi, A., Taylor, B. J. M., Nitsch, R., Lundin, A., Cavallo, A. L., Madeyski-Bengtson, K., Karlsson, F., Clausen, M., Hicks, R., Mayr, L. M. et al.(2018). Decoding non-random mutational signatures at Cas9 targeted sites. Nucleic Acids Res 46, 8417-8434.
Allen, F.R., Crepaldi, L.R., Alsinet-Armengol, C. ,Strong, A., Kleshchevnikov, V., Pietro De Angeli, P., Palenikova, P., Kosicki, M., Bassett, A.R., Harding, H. et al.(2018). Mutations generated by repair of Cas9-induced double strand breaks are predictable from surrounding sequence. bioRxiv.
Questions for Authors
Q1. The finding that the chromatin state of a particular target influences targeting efficiency is interesting as it is not obvious how you could incorporate this into existing prediction algorithms as the optimal sgRNA to knockout a gene in two different cell types is likely to be different. Do you think the indel patterns observed would be consistent in other cell lines, for example?
Q2. What are the implications for the progression of CRISPR-based technologies to the clinic if editing of the same target can vary between cell types or even between the same cell type in two patients?
Q3. Your preprint caused a stir on Twitter as it acknowledges Her Majesty Queen Elizabeth II for starting your sequencing run. Could you explain a bit more?
Posted on: 27 September 2018
doi: https://doi.org/10.1242/prelights.4951
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioinformatics category:
Expressive modeling and fast simulation for dynamic compartments
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
Spatial transcriptomics elucidates medulla niche supporting germinal center response in myasthenia gravis thymoma
Also in the molecular biology category:
Fetal brain response to maternal inflammation requires microglia
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Nanos2+ cells give rise to germline and somatic lineages in the sea anemone Nematostella vectensis
preLists in the bioinformatics category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |
Also in the molecular biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |











 (2 votes)
(2 votes)