Targeting light-gated chloride channels to neuronal somatodendritic domain reduces their excitatory effect in the axon
Preprint posted on 25 May 2018 https://www.biorxiv.org/content/early/2018/05/25/331165.1
Article now published in eLife at http://dx.doi.org/10.7554/elife.38506
High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins
Preprint posted on 8 December 2017 https://www.biorxiv.org/content/early/2017/12/08/225847
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-018-06511-8
Advanced soma-targeting of light-gated chloride channels delivers the most potent inhibitory optogenetic control to date.
Selected by Mahesh KarnaniCategories: cell biology, molecular biology, neuroscience, physiology
Summary
Recent advances have enhanced optogenetic inhibition by targeting a powerful light-gated chloride channel mostly to the somatodendritic compartment of neurons.
Context
Optogenetics, i.e., the use of light controlled membrane proteins to manipulate electrical activity of neurons, is a decisive technique in circuit neuroscience and beyond. Particularly the millisecond precision and cell specificity of this perturbation method enable profound advances in circuit studies. Neuronal activation is achieved routinely with channelrhodopsin variants to suit the illumination wavelength and speed required. However, neuronal inhibition with optogenetics is less trivial1. This is fundamentally due to the different transmembrane driving forces of ions available for inhibition compared to those for depolarisation. Reversal potentials of inhibiting anion channel currents are more varied and closer to a typical resting membrane potential than the reversal potentials of depolarising cation channel currents. This causes problems for inhibition. One problem is that chloride channels are commonly excitatory at axon terminals while being inhibitory in somatodendritic domains of mature neurons. In contrast, cation channels like channelrhodopsin will depolarise and elicit action potentials regardless of subcellular compartment. Besides channels, ionic pumps may be used to manipulate the membrane potential. This has been a useful strategy for optogenetic inhibition, and experimenters have greatly benefited from the light activated inward chloride pump halorhodopsin, and the outward proton pump archeorhodopsin. These come with some disadvantages though – the hyperpolarising current achieved with pumps is less effective at inhibition than inhibitory ion channels, and archeorhodopsin has an unforeseen side-effect of exciting some axons due to calcium influx triggered by increased intracellular pH2. For these reasons, the high conductance anion channelrhodopsin GtACR2 is a promising advance in optogenetic inhibition, if its expression can be excluded from axons where it would be excitatory.
Key findings
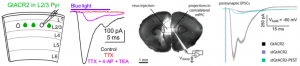
These preprints show that GtACR2 is excitatory in axons, in complementary sets of experiments mostly in cultured neurons and in vivo (Mahn et al.), and in brain slices with pharmacological manipulations (Messier et al.). Both preprints demonstrate light activated postsynaptic currents in neurons that are innervated by GtACR2 bearing fibers. Messier and colleagues went on to show these currents were sensitive to synaptic blockers and were abolished by action potential blockade with tetrodotoxin. The currents reappeared when 4-aminopyridine and tetraethylammonium were added on top of tetrodotoxin to prolong membrane depolarisation – a tell-tale sign of direct depolarisation of presynaptic terminals (see Figure). Both studies also observed antidromic (initiated in the axon) spikes during light induced hyperpolarisation in the somata of GtACR2 bearing cells, in line with axonal depolarisation.
Besides GtACR2 and its variant GtACR1, two other light-gated chloride channels, iC++ and iChloC, performed similarly, though GtACR2 was more powerful. This is why both studies selected GtACR2 for a further modification that targets it to the somato-dendritic compartment. Mahn and colleagues fused GtACR2 to the Kv2.1 C-terminal segment (Kv2.1C), a strategy that has been used in two recent publications3,4. Messier and colleagues screened eight different soma-targeting motifs, including Kv2.1C, and found greatest somatodendritic specificity with a hybrid targeting motif Kv2.1C-linker-TlcnC. Both studies found several-fold increased somatic inhibitory current and decreased axonal activation with their soma-targeting constructs.
Why I chose these preprints
I am a believer in the transformative power of optogenetics, but sometimes I note more seasoned researchers ‘stroking their beards’ tentatively when these beliefs are laid bare. I think this is because optogenetics is a relatively young technique and as such we are still unclear of all the caveats. The ambiguity of effects of inhibitory opsins has prompted a few studies before these preprints5,2. On the other hand, we might question how physiologically relevant it is to use excitatory opsins to activate a population of neurons at millisecond synchrony out of the blue from the circuit’s perspective. Might it be more normative for a circuit to gradually ramp up its activity and find its own resonant frequency than to receive a constant frequency pulse train? Is it relevant to average across repeated trials given that there are likely plasticity effects from such a powerful activation?
Another reason these studies are crucial is that targeting opsins exclusively to somata is the way forward for true single-cell resolution optical control3,4,6,7. It is clear from many studies that off-target activation of the neuropil structures (i.e., dendrites and axons) adjacent to somata is a caveat for single cell manipulation even with state-of-the-art optical systems. Sparse, soma-specific expression offers a solution.
What next?
These studies honestly and constructively address the challenges inherent in optogenetic inhibition. The authors have made considerable progress toward reliable optogenetic inhibition by targetting the opsin to somatodendritic domain. The achieved soma-specific expression is not total in either case however. Higher soma-specificity would be highly welcome, but how could this be achieved? Messier and colleagues mention engineering inward rectification to the chloride channel, seeking better soma-targeting motifs or simply reducing overall expression level of the current mostly soma-targeted protein such that the axonal activation would be minimal. One might imagine that for pyramidal neurons targeting opsin expression to the axon-initial-segment could yield further improvement. Another possibility may be some form of two-component strategy involving a soma-specific structure (nuclear membrane protein interacting with plasma-membrane?). On the other hand, just for reliable inhibition in general, a light-gated potassium channel might be optimal1, as the potassium equilibrium potential tends to remain hyperpolarised in axons.
References:
- Wiegert, J. S., Mahn, M., Prigge, M., Printz, Y. & Yizhar, O. Silencing Neurons: Tools, Applications, and Experimental Constraints. Neuron 95, 504–529 (2017).
- Mahn, M., Prigge, M., Ron, S., Levy, R. & Yizhar, O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat. Neurosci. 19, 554–556 (2016).
- Forli, A. et al. Two-Photon Bidirectional Control and Imaging of Neuronal Excitability with High Spatial Resolution In Vivo. Cell Rep. 22, 3087–3098 (2018).
- Mardinly, A. R. et al. Precise multimodal optical control of neural ensemble activity. Nat. Neurosci. 21, 881–893 (2018).
- El-Gaby, M. et al. Archaerhodopsin Selectively and Reversibly Silences Synaptic Transmission through Altered pH. Cell Rep. 16, 2259–2268 (2016).
- Shemesh, O. A. et al. Temporally precise single-cell-resolution optogenetics. Nat. Neurosci. 20, 1796–1806 (2017).
- Baker, C. A., Elyada, Y. M., Parra, A. & Bolton, M. M. Cellular resolution circuit mapping with temporal-focused excitation of soma-targeted channelrhodopsin. Elife 5, (2016).
Posted on: 22 July 2018
Sign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cell biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Structural basis of respiratory complexes adaptation to cold temperatures
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Also in the molecular biology category:
Clusters of lineage-specific genes are anchored by ZNF274 in repressive perinucleolar compartments
Nanos2+ cells give rise to germline and somatic lineages in the sea anemone Nematostella vectensis
Plant plasmodesmata bridges form through ER-driven incomplete cytokinesis
AND
Plasmodesmata act as unconventional membrane contact sites regulating inter-cellular molecular exchange in plants
Also in the neuroscience category:
Transcriptional profiling of human brain cortex identifies novel lncRNA-mediated networks dysregulated in amyotrophic lateral sclerosis
RIPK3 coordinates RHIM domain-dependent inflammatory transcription in neurons
Synergistic olfactory processing for social plasticity in desert locusts
Also in the physiology category:
Unlocking the secrets of kangaroo locomotor energetics: Postural adaptations underpin increased tendon stress in hopping kangaroos
Changes in surface temperatures reveal the thermal challenge associated with catastrophic moult in captive Gentoo penguins
Torpor energetics are related to the interaction between body mass and climate in bats of the family Vespertilionidae
preLists in the cell biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
Cell Polarity
Recent research from the field of cell polarity is summarized in this list of preprints. It comprises of studies focusing on various forms of cell polarity ranging from epithelial polarity, planar cell polarity to front-to-rear polarity.
| List by | Yamini Ravichandran |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |
BSCB/BSDB Annual Meeting 2019
Preprints presented at the BSCB/BSDB Annual Meeting 2019
| List by | Dey Lab |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |
ASCB/EMBO Annual Meeting 2018
This list relates to preprints that were discussed at the recent ASCB conference.
| List by | Dey Lab, Amanda Haage |
Also in the molecular biology category:
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
MitoList
This list of preprints is focused on work expanding our knowledge on mitochondria in any organism, tissue or cell type, from the normal biology to the pathology.
| List by | Sandra Franco Iborra |
Also in the neuroscience category:
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |
Also in the physiology category:
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (1 votes)
(1 votes)