Novel functions for integrin-associated proteins revealed by myofibril attachment in Drosophila
Posted on: 18 May 2018
Preprint posted on 6 April 2018
Article now published in eLife at http://dx.doi.org/10.7554/eLife.35783
Nicholas Brown and colleagues unravel some of the intricacies of integrin adhesion complex organisation and signalling by genetic manipulation, electron microscopy and super-resolution microscopy using the Drosophila myotendinous junction as a model
Selected by Jon Humphries
How this work fits the bigger picture
Cell adhesion to the extracellular matrix (ECM) is primarily mediated by the integrin family of cell surface receptors and is essential for many developmental and pathological processes. Cell-ECM adhesion nucleates the formation of multi-protein adhesion complexes at the interface of the plasma membrane and cytoskeleton that provide inputs into a wide variety of signalling pathways including mechanosensation, proliferation, survival and differentiation. Adhesion complexes comprise a large number of integrin-associated proteins (IAPs), however, specific roles for each IAP and how their functions are coordinated has yet to be fully clarified.
Key findings
In this preprint Green et al. use Drosophila muscle attachment sites as an in vivo model of adhesion complexes to study integrin-associated protein (IAP) function. In particular the authors utilised genetic ablation of selected IAPs, and the unique physiological features of Drosophila indirect flight muscles which are more similar to vertebrate muscles, to identify the function of four IAPs (tensin, RSU1, FAK and vinculin) that are not required for Drosophila viability. Moreover, through genetic removal of combinations of IAPs the authors demonstrated unanticipated positive and negative functional interactions between IAPs, with FAK inhibiting integrins via tensin, and RSU1 regulating the activity of PINCH. Furthermore, Green et al also used super-resolution microscopy to define the molecular organisation of IAPs at muscle attachment sites. By this approach the authors showed four distinct layers based on their composition, with both similarities and differences to the currently adopted model of the spatial arrangement of IAPs in adhesion complexes. The four regions defined were the integrin signalling layer (ISL) comprising membrane adhesion structures and most IAPs, a force transduction layer (FTL) containing actin and the C-terminus of talin and vinculin, a novel actin-rich region containing filamin, Arp3 and WASH which the authors term the muscle actin regulatory layer (MARL), and the first Z-line containing ZASP and α-actinin which is analogous to a stress fibre attached to a focal adhesion.
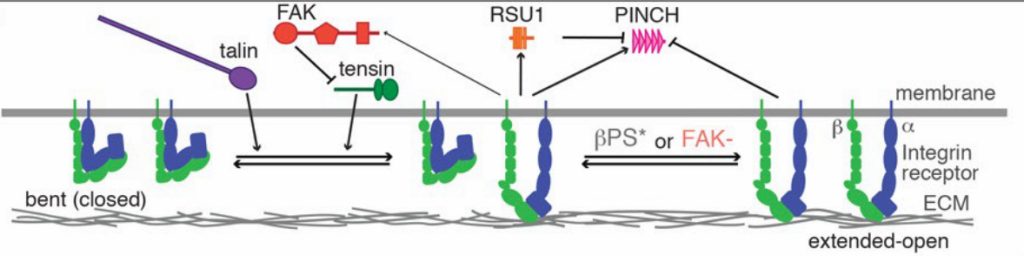
Model of IAP function. Adapted from Green et al. Figure 3B, made available under a CC-BY-NC-ND 4.0 license
Why I found this preprint interesting
Looking forward it will be interesting to see if other model organisms or tissues display similar IAP organisational or signalling properties. However, I was drawn to this manuscript for a number of reasons. Firstly, the ability to reveal and define functional roles and interactions for IAPs in a physiological context is a very powerful validation of the importance of cell adhesion biology that is commonly studied in mammalian cell culture models. Secondly, the authors make a valuable contribution to the field by providing an in vivo view of the molecular organisation of the adhesion complex at myofibril attachment sites by super-resolution microscopy. Finally, and from a very personal perspective, colleagues of mine at The University of Manchester have established a valuable resource for the use of Drosophila as tools for outreach activities in schools (https://droso4schools.wordpress.com/). Since I have personally found these resources to be useful with school outreach activities I am keen to promote the immense value fruit fly research has provided to the study of biology in general.
Read preprint










 (No Ratings Yet)
(No Ratings Yet)