Combinatorial patterns of graded RhoA activation and uniform F-actin depletion promote tissue curvature
Posted on: 30 April 2020
Preprint posted on 16 April 2020
Article now published in Development at http://dx.doi.org/10.1242/dev.199232
Patterns in the groove: Gradients of RhoA modulate F-actin patterns in Drosophila mesodermal invagination
Selected by Grace LimBackground
To ensure robust tissue morphogenesis, gene expression patterns and cell shape changes are coordinated across the tissue in a tightly regulated manner. These reproducible patterns of tissue morphogenesis are essential for proper development, as dysregulation of pathways controlling growth and cellular mechanics often result in impaired functionality and disease (Lecuit et al., 2011; Nelson and Gleghorn, 2012).
In this study, the authors investigated the mechanisms behind tissue folding during ventral furrow formation in the Drosophila mesoderm. Invagination of the mesoderm tissue at the ventral midline occurs via apical constriction of the ventral cells, a process driven by apical actomyosin contractility (Figure 1). This apical constriction event is characterized by the shrinking of apical cell areas, resulting in a wedge-shaped cell geometry in constricting cells (Martin and Goldstein, 2014). When situated within a larger adhesive tissue like the Drosophila mesoderm, apical constriction can influence global tissue architecture by causing tissue folding and the formation of higher-order structures such as the mesodermal tube.
In wildtype Drosophila embryos, a gradient of apical constriction is displayed along the ventral-lateral axis during ventral furrow formation (Figure 1). However, how this process is regulated on a tissue-wide scale is not well understood.
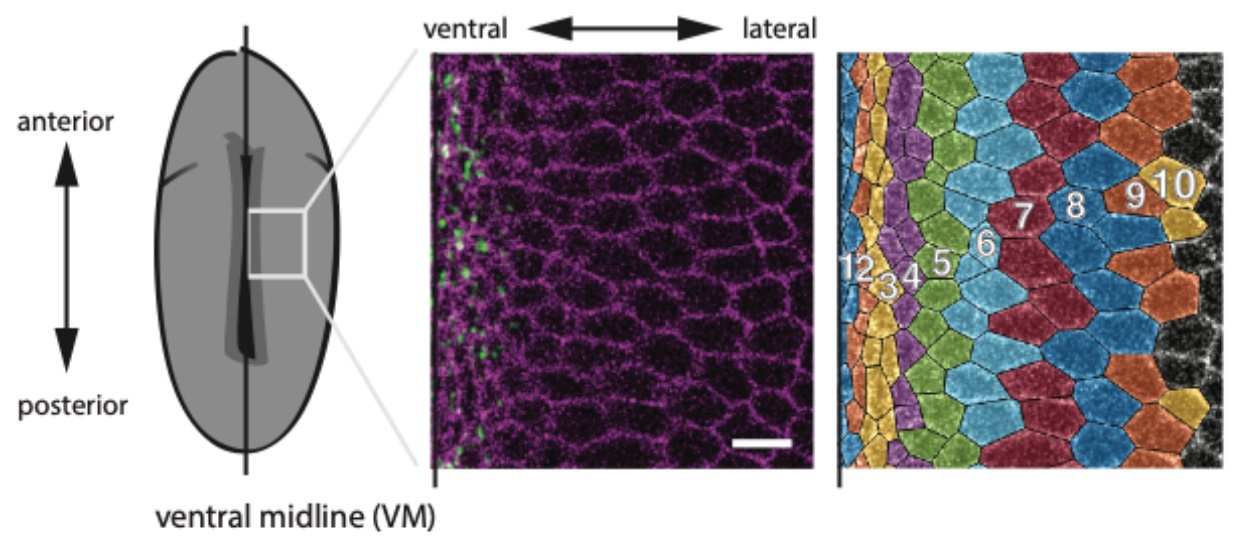
Figure 1: Schematic of the Drosophila embryo illustrating the position of the ventral midline (left). Confocal image of mesoderm cells around the ventral midline, with membranes marked in purple and myosin labelled in green (middle). Computationally segmented cells, with different colours for each cell row (right). Adapted from Figure 1A of Denk-Lobnig et al.
Key findings
To dissect how gradients of actomyosin contractility and apical constriction are established in the Drosophila mesoderm, the authors conducted a thorough investigation of tissue-wide expression patterns for actin, myosin, and their upstream regulators, both before and during tissue invagination. While myosin levels displayed a decreasing gradient laterally from the ventral midline (Figure 1), F-actin levels were initially low throughout mesoderm cells prior to tissue invagination (relative to ectoderm levels), but subsequently increased during tissue invagination in the cell rows closest to the ventral midline. Thus, during invagination, F-actin levels display a distinct pattern from myosin: a peak of F-actin in cell rows closest to the ventral midline, and a zone of F-actin depletion in rows away from the ventral midline.
The authors then went on to demonstrate that this precise F-actin patterning during invagination can be explained by the combined effect of the upstream transcription factors Snail and Twist. Snail is specifically expressed within the mesoderm where F-actin levels are lower than in ectoderm, and strikingly, snail mutants no longer display the distinct F-actin boundary between mesoderm and ectoderm, with uniform F-actin levels across both tissues. In contrast, Twist is expressed in a gradient in the mesoderm that is highest at the ventral midline and decreases laterally. Unlike the snail mutants, twist mutants showed a clear F-actin mesoderm-ectoderm boundary, but no longer sustained the F-actin gradient patterning about the ventral midline. Together, the data support a model whereby uniform mesoderm expression of Snail first acts to depress F-actin levels throughout the mesoderm, and the Twist gradient about the ventral midline subsequently increases F-actin levels in a similar gradient (Figure 2).
Interestingly, the width of the gradient of Twist mRNA about the ventral midline is larger than the gradients observed for myosin and F-actin, suggesting that other regulators could be at play. Downstream of Twist, the Rho pathway is known to promote actin polymerization and myosin activity, making it a probable candidate for regulating actomyosin patterns during tissue invagination. Consistent with this, the authors found that multiple members of the Rho pathway, including RhoGEF2, Anillin (RhoA sensor) and ROCK, all displayed gradients along the ventral-lateral axis. Furthermore, modulating the level of RhoA activity by manipulating the levels of Rho GAP and GEF was sufficient to perturb actomyosin patterns in the mesoderm, with increasing RhoA activity causing an expansion of the zone of higher F-actin and myosin levels around the ventral midline, and a corresponding shrinkage of the zone of F-actin depletion located more laterally. As with many other biological contexts where complex patterns are generated, the balance of multiple regulators like GAPs and GEFs for Rho activity appears to be a common theme (Denk-Lobnig and Martin, 2019).
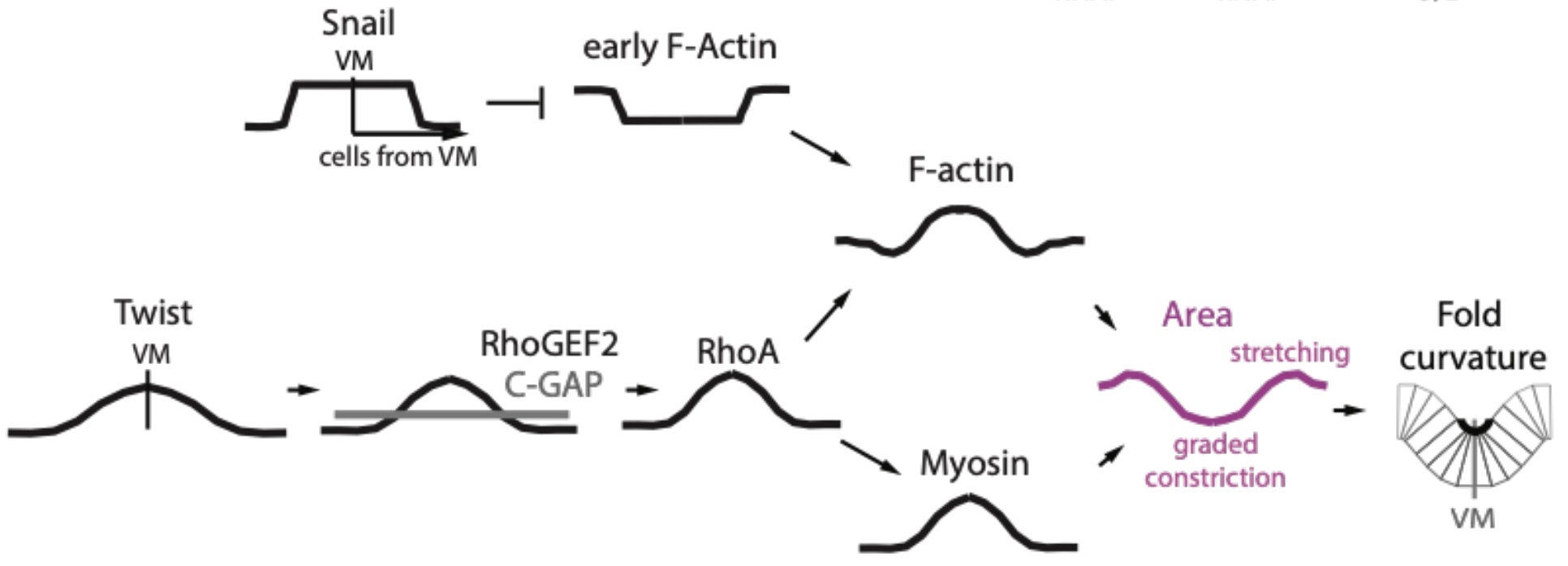
Figure 2: Summary of key regulators generating distinct patterns of F-actin and myosin
within the Drosophila mesoderm during ventral furrow formation. Adapted from Figure 7E of Denk-Lobnig et al.
Finally, the relevance of the precise actin and myosin gradients for ventral furrow formation was tested using the Rho activity manipulations described above. In line with actomyosin-driven apical constriction inducing cell shape changes to drive tissue curvature, the authors found that altering the precise actomyosin gradients set up in the mesoderm indeed affected the central curvature of the tissue at the ventral midline and the resultant size of lumen following mesodermal tube formation (Figure 3). While wild-type embryos formed a sharp V-shaped fold during invagination (Figure 3A), the expansion of the actomyosin gradient by elevating RhoA activity resulted in a wider, C-shaped fold (Figure 3B). Together, the results demonstrate how distinct patterns of Snail and Twist acting through RhoA can collectively regulate the actomyosin gradient across the ventral-lateral axis during mesoderm invagination to ensure proper tissue curvature and tube formation.
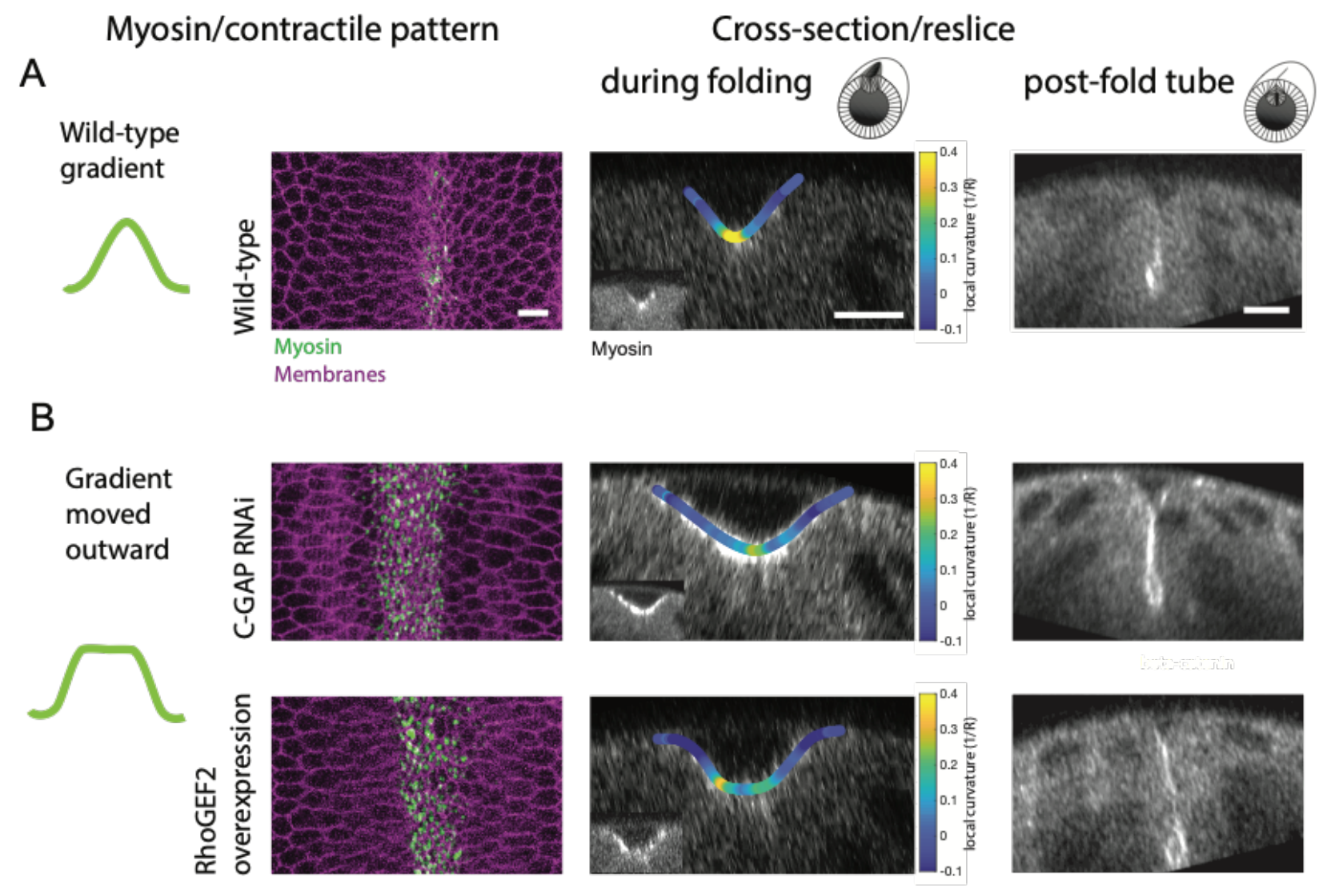
Figure 3: (A) Wild-type embryos display a myosin gradient about the ventral midline resulting in a sharp, V-shaped fold during ventral furrow formation and a mesodermal tube. (B) Expanding the myosin gradient outward using Rho GAP RNAi or overexpression of RhoGEF2 produced a wider C-shaped fold during ventral furrow formation and a larger post-fold tube. Adapted from Figure 7A, B of Denk-Lobnig et al.
What I like about this preprint
This preprint stands out for the careful dissection of tissue-wide patterns, bringing together multiple molecular players to understand how apical constriction is precisely regulated beyond the cellular scale. Although the role of actomyosin contractility in driving apical constriction about the ventral midline has been studied previously (Heer et al., 2017), in this work, the authors further demonstrate that actin and myosin gradients are in fact distinct from one another, with separate zones of actin clearance and actin gradients that could explain the gradients in apical constriction across the mesoderm tissue. Importantly, this spatial characterization of actin levels then formed the basis in connecting multiple upstream regulators that work in combination to create the precise patterns of actin and apical constriction in the tissue. While the molecular pathways regulating actin and myosin patterns remain incompletely elucidated – for instance, how RhoA controls actin regulators like formins – this study sets the stage for future work in understanding how different regulators can work in concert to produce complex developmental patterns and structures, both in the Drosophila mesoderm and in other systems.
Future directions and questions for the authors
- The authors elegantly show with their Rho GAP/GEF manipulations that the balance of GAP and GEF activities can modulate the extent of myosin and F-actin gradients in the mesoderm. Does actin also feedback onto Rho activity in the mesoderm, as seen in other systems?
- The ectoderm phenotypes were used as a reference point in this study but not directly investigated. The authors demonstrated that ectoderm cells sustained high F-actin levels before and during furrow formation, and maintained their apical cell areas throughout this process. Is there a role for F-actin in the ectoderm in buffering against cell shape changes occurring in the adjacent mesoderm cells?
References
Denk-Lobnig, M., and Martin, A.C. (2019). Modular regulation of Rho family GTPases in development. Small GTPases 10, 122–129.
Heer, N.C., Miller, P.W., Chanet, S., Stoop, N., Dunkel, J., and Martin, A.C. (2017). Actomyosin-based tissue folding requires a multicellular myosin gradient. Development 144, 1876–1886.
Lecuit, T., Lenne, P.-F., and Munro, E. (2011). Force Generation, Transmission, and Integration during Cell and Tissue Morphogenesis. Annu. Rev. Cell Dev. Biol. 27, 157–184.
Martin, A.C., and Goldstein, B. (2014). Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141, 1987–1998.
Nelson, C.M., and Gleghorn, J.P. (2012). Sculpting Organs: Mechanical Regulation of Tissue Development. Annual Review of Biomedical Engineering 14, 129–154.
doi: https://doi.org/10.1242/prelights.19768
Read preprint










 (No Ratings Yet)
(No Ratings Yet)