The creation of sexual dimorphism in Drosophila gonad stem cell niches
Posted on: 4 August 2018
Preprint posted on 11 July 2018
Battle of the Sexes: Doublesex tips the balance between male and female programs for gonadal stem cell niche development
Selected by Natalie DyeWhy I chose this work:
A fundamental open question in biology is how organisms achieve system-wide coordination in morphology and developmental timing. Sexual dimorphism – the emergence of sex-specific morphologies and behaviors – is a great example of such coordination. The mechanisms determining sex must influence morphological development in many different tissues in different ways. The open questions are not unlike those that I think about in the context of hormone signaling. For example, at metamorphosis in the fly and puberty in mammals, steroid signaling triggers many different types of cellular events, depending on the tissue. The emergence of specificity from a single trigger must involve integration with tissue-specific signals in ways that are still poorly understood.
Background:
The sex-specific development of the gonad is perhaps the most important example of sexual dimorphism. As in many tissues, sexual dimorphism in the gonad depends on Doublesex (Dsx), a widely-conserved transcription factor regulating sexual development in many organisms. Male and female flies produce distinct functional splice isoforms of Dsx, resulting from female-specific expression of Sex lethal, a master regulator of sex determination.
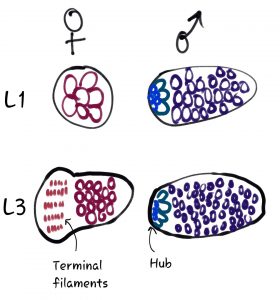
The authors of this preprint use the Drosophila model to address key unknowns about how and when Dsx influences the sex-specific development of the gonad stem cell niche (the “hub” in males and the “terminal filaments” and “cap cells” in females, see Fig 1). Previous work showed that the gonads of both XX and XY animals of dsx mutants begin developing along a male path during embryogenesis, forming 3 male-specific cell types. Male and female gonads naturally develop at different timescales, however, with males developing earlier than females. Here, by following these dsx mutants through later development, the authors gain key novel insight into the function of Dsx.
New findings:
1. Dsx is not absolutely required to form either the male or female gonad stem cell niches; rather it seems to bias an otherwise stochastic choice between these two developmental programs, so that the whole organism has the same sex-appropriate structures.
- Dsx mutants do not form an intersex or poorly developed niche – rather they fully develop as either male or female in a seemingly random fashion: both XX and XY dsx mutant adults have ~50% male and ~50% female stem cell niches.
- Within a given gonad, all cells exhibit the same sex-specific gene expression; but two gonads of the same individual can differ in whether they have a male-specific hub or female-specific terminal filaments and cap cells.
2. Dsx makes the decision between male and female niche programs robust and irreversible. The timing of this action differs between the sexes, however, with males becoming fixed before the 2nd instar, whereas females remain plastic until late larval stages.
- While previous work showed that dsx mutants start developing male-specific niche cell types, here we see that in the absence of Dsx, the developing gonad is actually intersexual and rather plastic. They express an intermediate level of certain genes that are normally highly sex-specific, and roughly half of the initially male-like structures transform into female structures by proliferating in the 2nd – 3rd instars.
- In contrast, male hubs that form in the presence of the male Dsx isoform do not transform into female structures, even if we forcibly switch Dsx to the female isoform in the 2nd instar (using a temperature-sensitive allele of Tra2). Thus, without Dsx, the gonads are plastic and able to switch in the 2nd instar, but the presence of male Dsx fixes them robustly in a male program sometime before that.
- When starting with a female-specific Dsx isoform, the decision is not fixed until much later – forcing the production of male Dsx in the 2nd instar is able to almost randomize the production of male and female gonads. Only by pupal stages is the production of male Dsx unable to change the sexual fate of the gonads.
3. Bric-a-brac 1 and 2 are two related transcription factors acting downstream of Dsx in gonal stem cell niche development.
- Bric-a-brac 1 is only expressed in the female-specific terminal filaments, only at the onset of female gonad stem cell niche development (3rd instar).
- Nonetheless, both bric-a-brac genes are required for female-specific terminal filaments to form in a dsx mutant: removing either gene individually or one copy of both genes in a dsx mutant is sufficient to produce far more male structures than female structures.
Outlook for future work:
This work indicates that Dsx is not “micro-managing” gonad niche development but simply biasing the choice between two independent genetic programs. Now the interesting question is how it does that. Identifying downstream effectors like Bric-a-brac is a good start.
Another interesting finding that seems worth pursuing is that cells of a given gonad still make a “group decision” about what sex to be in the absence of Dsx. What is the basis of this coordination? Are they signaling to one another, and if so, by what pathway?
Lastly, perhaps the most challenging future direction will be the one addressing the observed sexual differences in developmental timing – why do female gonad stem cell niches remain plastic for longer than males? What times the onset of female gonad development? Could it involve ecdysone, the fly steroid, whose levels start gradually increasing at that time? Also, I would find it interesting to know how those male hubs transform into female structures in dsx mutants during the 2nd-3rd instars – what triggers their proliferation and how does that morphogenesis occur?
Looking forward to your future work, van Doren lab.
doi: https://doi.org/10.1242/prelights.4079
Read preprint










 (No Ratings Yet)
(No Ratings Yet)