Plasmodesmal connectivity in C4 Gynandropsis gynandra is induced by light and dependent on photosynthesis
Posted on: 30 January 2023
Preprint posted on 13 December 2022
Categories: plant biology
Background:
Plasmodesmata are channels that connect adjacent cells to link plasma membrane, cytoplasm, and endoplasmic reticulum for intercellular material exchange (Heinlein, 2002). Warm-season (C4) grass relies on plasmodesmata to exchange metabolites between mesophyll and bundle sheath (BS) cells but maintains spatial separation of carboxylation and decarboxylation in adjacent areas. In this preprint, Schreier and colleagues show that a certain C4 dicotyledons, Gynandropsis gynandra, is equipped with more plasmodesmata in M-BS interface than their C3 phylogenic relatives in a photosynthetic-dependent manner.
Key finding in this preprint:
(1) C4 dicotyledons have more plasmodesmata in the M-BS interface compared to C3 plants
The authors quantified plasmodesmata connections using electron microscopy in the C4 dicotyledon Gynandropsis gynandra and their C3 relatives Tarenaya hassleriana and Arabidopsis thaliana. They clearly showed higher plasmodesmata numbers in the M-BS interface of G. gynandra than could be found in both C3 species (Fig. 1), but no obvious differences in M-M and BS-BS interfaces in all species.
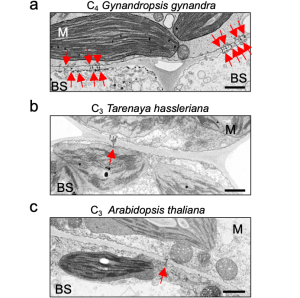
Figure 1. The M-BS cell interface of mature leaves of C4 Gynandropsis gynandra (left panel) has more plasmodesmata than closely related C3 species, Tarenaya hassleriana (middle panel) and Arabidopsis thaliana (right panel). Modified from Fig. 1 of (Schreier et al., 2022).
(2) Photosynthetic competence of chloroplasts is essential for light-induced plasmodesmata formation
The contributions of light and photosynthesis on plasmodesmata formation in the M-BS interface of the de-etiolating dicotyledon was not known yet. The authors first observed higher plasmodesmata formation in 24-h light treated de-etiolating seedlings (Fig. 2, right panel) compared to etiolating counterparts (Fig. 2, left panel) in the M-BS interface. Next, the authors triggered chloroplast developmental defects by chemical reagents leading to a reduction in PSII activity and plasmodesmata frequency in the M-BS interface, while blockage of electron transport via DCMU decreased the plasmodesmata formation. This could be fully rescued by the presence of photosynthate sucrose (Fig. 3).

Figure 2. Light acts as a developmental cue to increase plasmodesmata formation at the M-BS cell interface in cotyledons of C4 G. gynandra. Scanning electron micrographs of the M-BS interfaces in C4 G. gynandra cotyledons in etiolated (0 h, left panel) and de-etiolated (24 h, right panel) seedlings are shown. Red arrows indicate individual plasmodesma. Modified from Fig. 4b of (Schreier et al., 2022).
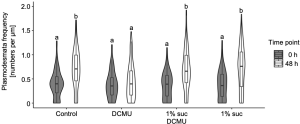
Figure 3. Sucrose rescues DCMU-mediated inhibition of plasmodesmata formation at the M-BS interface. Modified from Fig. 6 of (Schreier et al., 2022).
Why I chose this preprint
As a non-anatomist, I’m primarily interested in electron microscopy for its ability to visualize the ultrastructure of plasmodesmata in the plant cell interface. I enjoyed this preprint because it made me reconsider the multiple effects of light on plant development at different scales, from the level of the organism (photomorphogenesis) to the organelle (etioplast to chloroplast transformation). I also appreciate the massive number of images that are required for quantitative analysis of plasmodesmata frequency in C4 or C3 plants and different combinations of M or BS cell interfaces.
Questions for the authors
1. It’s surprising to me that we don’t know the plasmodesmata frequency in the C4 dicotyledon in contrast to monocot counterparts. Why do you think this is the case?
2. You showed that light and corresponding photosynthate triggers the formation of plasmodesmata in M-BS interface in de-etiolating C4 dicot seedlings. Is this formation a result of light-induced photomorphogenesis that initiates gene programming for plasmodesmata connections between M and BS cells?
3. How does photosynthate, like sucrose, coordinate plasmodesmata formation? Does it act as a signaling molecule to initiate cellular programming or as end products that reflect photosynthesis activity to synchronize plasmodesmata development in the right place?
References
Heinlein, M. (2002). Plasmodesmata: dynamic regulation and role in macromolecular cell-to-cell signaling. Curr Opin Plant Biol, 5(6), 543-552. doi:10.1016/s1369-5266(02)00295-9
Schreier, T. B., Müller, K. H., Eicke, S., Faulkner, C., Zeeman, S. C., & Hibberd, J. M. (2022). Plasmodesmal connectivity in C4 Gynandropsis gynandra is induced by light and dependent on photosynthesis. bioRxiv, 2022.2012.2007.519530. doi:10.1101/2022.12.07.519530
doi: https://doi.org/10.1242/prelights.33559
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the plant biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Actin Counters Geometry to Guide Plant Cell Division
Jeny Jose
The nucleus follows an internal cellular scale during polarized root hair cell development
Jeny Jose
preLists in the plant biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
The Society for Developmental Biology 82nd Annual Meeting
This preList is made up of the preprints discussed during the Society for Developmental Biology 82nd Annual Meeting that took place in Chicago in July 2023.
| List by | Joyce Yu, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)