Preprints by preLighters – David Wright
7 May 2020
In this new post series, we feature early-career researchers on the preLights team who have recently posted a preprint. They explain the main findings of their work, and why they chose to communicate their research through preprints.
First up we have David Wright, who is a postdoctoral researcher at the University of Leeds, where he is studying the function (and disfunction) of membrane proteins in disease. He has a keen interest in new technologies, and has written several preLights about new advances in CryoEM. Here, he talks about his recent work that was posted on bioRxiv:
Cryo-EM structures of human TRPC5 reveal interaction of a xanthine-based TRPC1/4/5 inhibitor with a conserved lipid binding site
David J. Wright, Katie J. Simmons, Rachel M. Johnson, David J. Beech, Stephen P. Muench, Robin S. Bon
https://www.biorxiv.org/content/10.1101/2020.04.17.047456v1

Could you describe the main findings of your preprint?
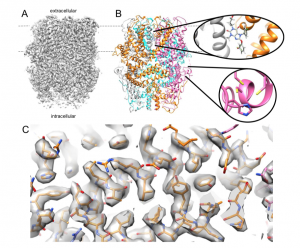
TRPC1/4/5 channels have been implicated in a variety of diseases, including anxiety and depression, kidney disorders, and cardiovascular diseases, and inhibition of these channels is a potential therapeutic strategy. Several small-molecule inhibitors have been developed, but their further development has been hindered by a lack of information on how they work on the molecular level. We successfully used cryogenic electron microscopy (cryoEM) to determine the structure of the TRPC5 channel in complex with the most potent TRPC1/4/5 inhibitor, Pico145. Our preprint is the first report of the identification of a small molecule binding site in any TRPC1/4/5 channel. An important observation from this work is that Pico145 replaces a lipid molecule that is bound in the TRPC5 channel when no Pico145 is present. This suggests that the binding site we found may be involved in lipid-mediated regulation of TRPC1/4/5 channels, a process that is still incompletely understood. This conserved lipid site has also been noted as a small molecule binding site in the related TRPC6 channel. We also identified a putative zinc binding site, which is conserved within the TRPC family and may be responsible for the reported channel gating by zinc ions. We expect that our work will aid the understanding of the regulation of TRPC1/4/5 channels and underpin the future design of specific and effective drugs for multiple diseases.
Why did you decide to post your work on bioRxiv?
Preprinting a manuscript is a fantastic way to disseminate new data, allowing the information to be freely available almost instantaneously. In our case, we had written our paper (which we have also submitted) and since the peer review process takes anywhere from a month to over a year, we felt it was important to make our data available for others to view as soon as possible. We felt that dissemination was especially urgent given the therapeutic potential of this ion channel, and the strong interest in this family of inhibitors. One of the great things about preprinting is that it immediately leads to discussions within the scientific community, which can inform and inspire later work.










