An asymmetry in the frequency and position of mitosis in the epiblast precedes gastrulation and suggests a role for mitotic rounding in cell delamination during primitive streak epithelial-mesenchymal transition
Posted on: 12 March 2020
Preprint posted on 21 February 2020
Article now published in EMBO reports at http://dx.doi.org/10.15252/embr.202050944
Regional differences in cell division within the gastrulating mouse embryo
Selected by Claire Simon & Sophie MorganiBackground:
Mitotic division, whereby a cell replicates its genetic material and splits into two daughter cells, is involved in tissue growth, homeostasis and repair. Cell division is not merely a “housekeeping” process but can drive morphogenesis, regulate cell fate decisions and influence the mechanical properties of tissues. In this pre-print, Despin-Guitard et al. characterize the position, orientation and frequency of cell divisions during mouse gastrulation, a critical developmental process when the pluripotent epiblast cells of the embryo give rise to and pattern the definitive germ layers.
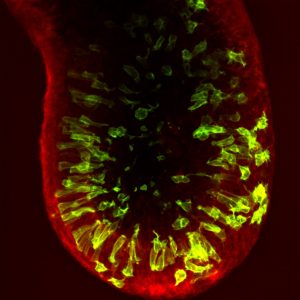
The gastrulating mouse embryo has a cylinder-like structure comprising a central cavity surrounded by the epiblast epithelium, which is encased by an outer visceral endoderm layer (see image). In almost all cases, cell division occurs at the apical surface of the epiblast, adjacent to the cavity. However, the authors show that, in contrast to what is observed in other pseudostratified epithelia, apically dividing epiblast cells do not maintain contact with the basal surface. This loss of basal anchoring could facilitate the extensive cellular rearrangements that occur during gastrulation.
Furthermore, the authors find that epiblast mitotic divisions occur at distinct apical-basal positions and frequencies in different regions of the embryo. While the majority of cell divisions are apical, prior to the onset of gastrulation within the posterior of the embryo a proportion of cells within the posterior of the embryo (where gastrulation will be initiated), undergo non-apical divisions. As cell division impacts cell fate, cell-cell adhesion and morphogenesis, it is tempting to speculate that these regional differences in cell division play a role during gastrulation. Conceivably, non-apical divisions could exert forces on the posterior outer visceral endoderm layer, triggering the activation of key gastrulation regulators and signals, such as Brachyury or the Wnt pathway, both of which are mechanosensitive [1]. Supporting this hypothesis, before gastrulation Wnt3 is expressed in a localized region of the VE overlying the posterior epiblast.
As the pluripotent cells begin to differentiate, they undergo an epithelial-mesenchymal transition (EMT) and exit the posterior epiblast epithelium in a region known as the primitive streak. Despin-Guitard et al. show that cells within the primitive streak exhibit an elevated frequency of mitotic division. As division increases the propensity of cells to delaminate from an epithelium [2], the authors hypothesize that, alongside EMT, increased cell division could encourage delamination from the epiblast.
Why we like this pre-print:
While gastrulation is one of the most critical developmental processes, it occurs after the embryo has implanted into the uterus of the mother which, historically, has made it challenging to visualize these events. As a result, we know little about how gastrulation is regulated over time. Recent advances in live imaging and ex vivo embryo culture have now opened the door to interrogate mammalian gastrulation in detail. This pre-print begins to address some of the key unknown questions, using a combination of live imaging and mosaically labelled mouse embryos to study cell division at gastrulation and many interesting questions arise from their data!
Open questions:
- Do anterior-posterior differences in cell division play a role in gastrulation?
- What causes posterior-specific non-apical cell divisions? Could the underlying basement membrane have started to break down before gastrulation, permitting basal division? Alternatively, could these differences be driven by the distinct anterior-posterior signalling environments?
- Do apical vs. non-apical cell divisions affect the mechanical properties of their neighbours and, as a consequence, do anterior and posterior embryonic regions have distinct mechanical properties?
- Can cells undergo mitosis-driven delamination from the epiblast without an EMT? If so, does this affect their ultimate cell fate?
NB: The phase of the cell cycle that cells occupy when exposed to a differentiation cue influences their cell fate [3]. Therefore, cells delaminating from the epiblast at mitosis might specify distinct cell types than those that undergo an EMT and exit the epiblast at a different stage of the cell cycle. This is particularly interesting when thinking about regions of the primitive streak where distinct cell types are generated within the same environment, such as the anterior primitive streak where both definitive endoderm and axial mesoderm arise.
doi: https://doi.org/10.1242/prelights.17560
Read preprint










 (No Ratings Yet)
(No Ratings Yet)