CXCR3-expressing metastasis-initiating cells induce and exploit a fibroblast niche in the lungs to fuel metastatic colonization
Posted on: 5 March 2019 , updated on: 25 March 2019
Preprint posted on 11 February 2019
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-020-15188-x
A new place to call home: disseminated cancer cells fleece lung fibroblasts to fuel a metastatic niche
Selected by Julija Hmeljak
BACKGROUND
Despite significant progress, metastasis remains the leading cause of mortality in cancer patients. Acquiring the ability to successfully metastasise to distal sites is a crucial, and not yet completely understood, stage in a cancer’s evolution. To do so, cancer cells must successfully leave the primary tumour site, survive in the bloodstream, invade the receiving tissue and establish a colony from which a new complex tumour will arise (Blomberg et al., 2018). The final step in this process, establishing a cancer cell colony within a secondary organ, requires significant changes to both the disseminated cancer cells and to the receiving stroma. A new preprint by the Oskarsson group explores the molecular crosstalk that leads to the successful establishment of breast cancer metastasis in lung tissue.
KEY FINDINGS
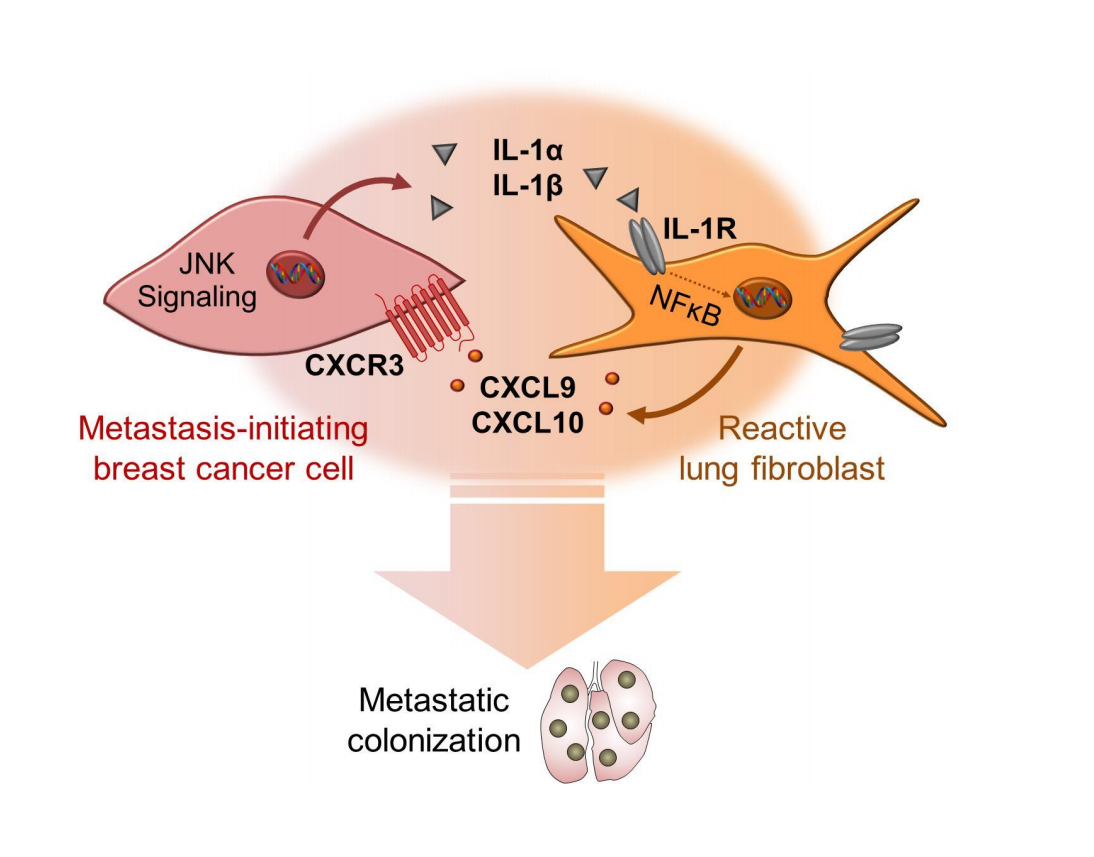
The authors injected GFP/luc-tagged breast cancer cells into the bloodstream of immunodeficient mice to induce lung metastases. They then performed a series of elegant experiments to show that metastasizing (disseminated) breast cancer cells evoke significant changes to lung fibroblasts, resulting in a niche that supports the establishment of a secondary tumour. These changes are mediated via an inflammatory signalling loop, whereby a specific subset of disseminated breast cancer cells excrete interleukin-1α and 1β to activate lung fibroblasts and push them towards a tumour-supportive phenotype, mainly by triggering an IL-1 receptor signalling-mediated expression of the chemokines CXCL9 and 10. Intriguingly, the same subset of disseminated breast cancer cells expresses the CXCR3 receptor, which binds CXCL9/10, indicating that metastasis-initiating cells are able to both induce and respond to molecular changes in reactive fibroblasts. This paracrine signalling remodels the receiving stroma and promotes the successful establishment of breast cancer metastases in the lung.
WHY I CHOSE THIS PREPRINT
The authors have built upon their previous work on the role of JNK signalling in breast cancer metastasis (Insua-Rodriguez et al., 2018), and have performed rigorous and well-controlled experiments providing interesting mechanistic insight into the cancer cell-stroma crosstalk. Studies of fibroblast interactions in the tumour microenvironment have predominantly focused on primary tumours (LeBleu and Kalluri, 2018). However, this work explores a less well understood aspect of the tumour microenvironment – the metastatic niche.
FUTURE PROSPECTS AND OPEN QUESTIONS
As the authors discuss in their conclusion, the role of CXCR3 signalling in cancer is complex, as it modulates the immune microenvironment. Although directly blocking the CXCR3 receptor may reduce the metastatic potential of this subset of highly metastatic breast cancer cells, it may have unintended consequences for T cell function and for antitumour immunity.
Are there any known actionable targets in the signalling pathway downstream of CXCR3?
Do you know if there are any recurrent (activating) mutations in CXCR3 in human cancers (e.g. from ICGC/TCGA cohorts)?
References:
Blomberg et al. (2018) Immune regulation of metastasis: mechanistic insights and therapeutic opportunities. Dis Model Mech. Oct 24;11(10).
Insua-Rodríguez Jet al. (2018) Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol Med. Sep 6: e9003.
LeBleu and Kalluri (2018) A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. Apr 19;11(4).
doi: https://doi.org/10.1242/prelights.8902
Read preprint










 (No Ratings Yet)
(No Ratings Yet)