Mechanism of cell polarisation and first lineage segregation in the human embryo
Posted on: 6 October 2020
Preprint posted on 23 September 2020
Background
During the earliest stages of embryonic life, the single-celled fertilized egg undergoes several rounds of cleavage divisions to form a 32- to 64-cell blastocyst – the first tissue-like architecture during development. The blastocyst comprises a well-differentiated outer epithelial layer known as the trophectoderm (the lineage that will form the placenta), which surrounds a pluripotent inner cell mass (the lineage that will form the embryo and other extraembryonic tissues) and a fluid-filled cavity. Critically, proper blastocyst formation and specification of blastocyst lineages are essential for the successful implantation of the blastocyst into the uterine wall, and early failures in these morphogenetic events have been thought to account for a large proportion of pregnancies that do not make it to term. This underscores the importance of understanding preimplantation embryo development to better improve pregnancy outcomes and IVF technologies.
The primary mammalian model for studying early embryo development has traditionally been the mouse embryo, whose overall morphological similarities to human embryos combined with their accessibility and amenability to ex vivo culture and experimental manipulations have made them ideal candidates for uncovering key pathways during embryo development (White et al., 2018). Studies over the past two decades have made significant inroads into many of the key molecular players involved in pathways driving fate specification. Yet precisely how these findings from the mouse embryo can be translated to an understanding of human embryo development has been visibly lacking.
Here, comparing key pathways during lineage specification in the human and mouse embryo, Zhu et al. identify a polarity-dependent mechanism promoting trophectoderm specification that is conserved between human and mouse embryos, providing important insights into the beginnings of human life.
Key findings
To understand how lineage segregation takes place in the human embryo, the authors first characterized how the early human embryo polarizes to set up the first apical and basal domains in development. The role of apical polarity in regulating trophectoderm fate via the Hippo pathway has been well established in the mouse embryo (Sasaki, 2015), but whether a parallel mechanism exists in the human embryo is unknown. Using a combination of immunofluorescence and time-lapse imaging, the authors dissect for the first time the initial steps leading to polarization of the human embryo. Strikingly, they find that the human embryo polarizes in two key steps: F-actin accumulates first at the apical surface, followed by the Par complex member PARD6 (Figure 1). This bears close resemblance to the sequence of events of cell polarization in the early mouse embryo, whereby the initial enrichment of actomyosin at the apical membrane is required for the subsequent polarization of Par complex components (Zhu et al., 2017).
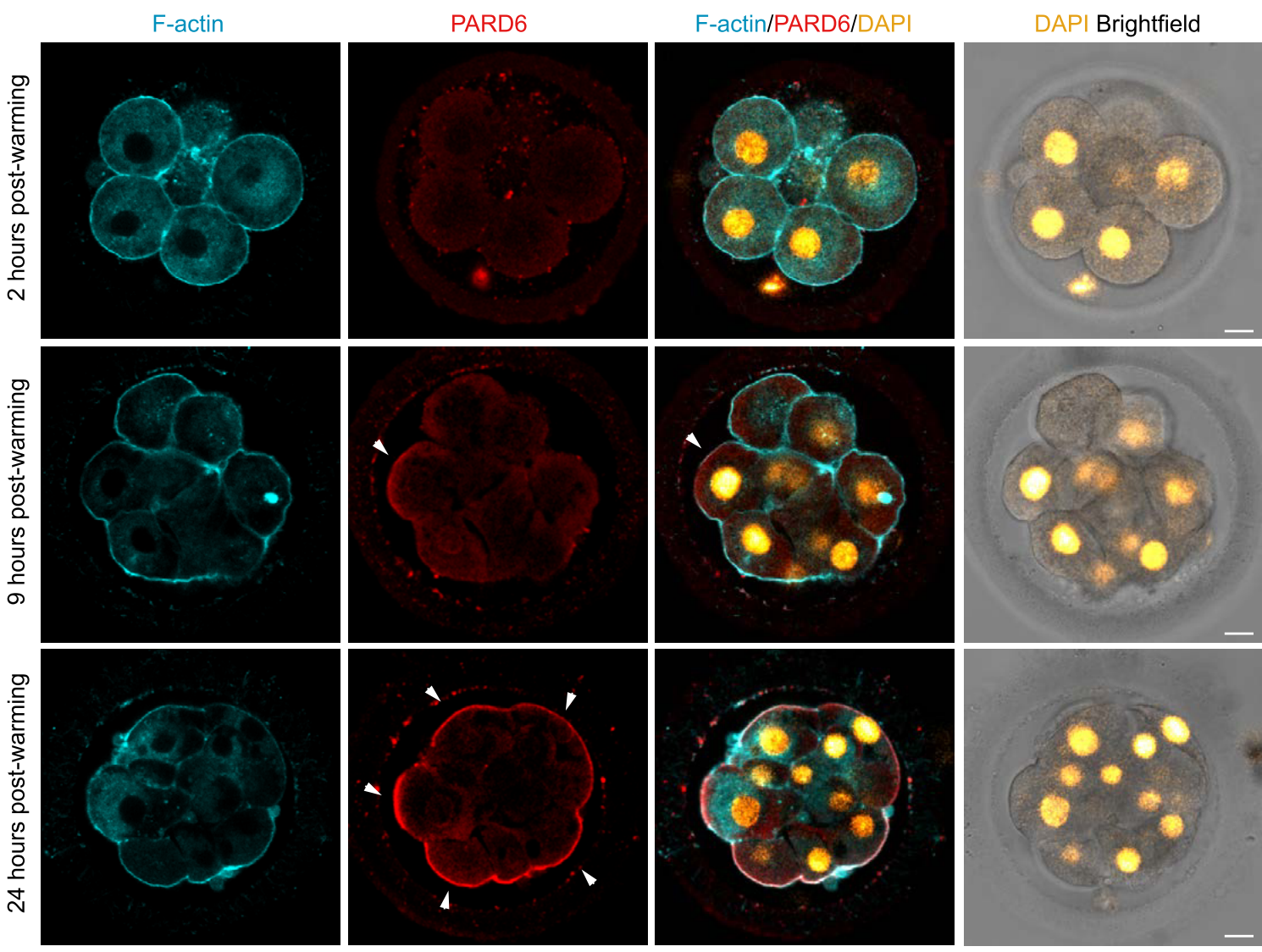
Figure 1: Patterns of F-actin and PARD6B localization in the early human embryo during embryo compaction and polarization. F-actin polarizes first to the apical surface, followed by PARD6B. Adapted from Fig. 1B of Zhu et al.
Polarization is accompanied by a major morphological change during early embryo development – compaction, a process whereby cells flatten up against their neighbours and increase their basolateral cell-cell contacts to produce a spherical mass. As a reliable measure of embryo compaction, the authors quantified the inter-blastomere angle between neighbouring blastomeres in compacting human embryos: Flatter surfaces in compacted embryos yielded larger inter-blastomere angles, whereas the rounder cells in uncompacted embryos displayed smaller angles. While it is known that the mouse embryo begins and completes the entire compaction process during the 8-cell stage of development, the authors found that compaction in the human embryo is instead prolonged, extending beyond the 8-cell stage to the 12-cell stage. Thus, human and mouse embryos polarize in the same manner but display key differences in their timing of compaction.
Given the striking similarities in polarization between human and mouse embryos, the authors next investigated whether human embryos also rely on PLC-PKC signalling to initiate polarization, as is the case in mouse embryos (Zhu et al., 2017). Two independent assays were used to perturb PLC activity: culturing the human embryos in the presence of the PLC inhibitor U73122, and downregulation of PLC expression in human embryos using RNAi. Both methods resulted in a significant reduction in polarized cells and diminished expression of PARD6 in apical membranes, demonstrating that PLC activity regulates cell polarization in the early human embryo.
Finally, the authors explored the relationship between polarization and lineage specification in the context of the human embryo, focusing on the spatiotemporal expression patterns of one of the primary trophectoderm lineage markers, GATA3. As expected, polar outer cells expressed higher levels of GATA3 compared to apolar inner cells of the embryo. Moreover, culturing human embryos in U73122 to inhibit PLC activity and cell polarization reduced GATA3 levels, although the total number of GATA3+ cells in the embryo remained constant. Importantly, despite being a trophectoderm marker, GATA3 was detected in both polar and apolar cells of day 4 human embryos. In all, these results indicate that the initiation of GATA3 expression occurs independently of polarity in both outer and inner cells, but a polarity-dependent mechanism reinforces GATA3 expression specifically within outer cells of the human embryo to direct them towards the trophectoderm lineage.
What I like about this preprint
The study of human preimplantation development holds great potential for understanding how life for us begins, and how to tap on this knowledge to improve fertility and IVF treatment outcomes. While it remains difficult to conduct extensive human embryo studies owing to the limited supply of donated embryos and ethical considerations, studies like Zhu et al. are a step in the right direction, providing a rare glimpse into the earliest stages of human development. In exploring trophectoderm lineage specification, the authors reveal key similarities and differences in the timing and manner of polarization in the human and mouse embryo, and convincingly establish a crucial role for polarity in reinforcing trophectoderm lineage identity in polarized outer cells of the human embryo. Importantly, their work also corroborates recent findings of a polarity-dependent mechanism driving trophectoderm specification that is conserved between human, mouse, and cow preimplantation embryos (Gerri et al., 2020). These studies, together with continued investigations into comparative embryology, will help to further illuminate key conserved mechanisms across mammalian embryonic development.
Questions for authors
- During polarization of the mouse embryo, apical localization of the Par complex results in the exclusion of actin from the centre of the apical surface, forming a mature actin ring/domain. Do the authors observe a similar “actin ring” structure at the apical surface of early human embryos? If so, can the authors speculate if this ring forms by a similar mechanism as in mouse embryos?
- Apart from downregulating PLC activity, have the authors tried the converse experiment to hyperactivate PLC-PKC signalling in the human embryo, to see if they can induce premature polarization and/or compaction?
- The authors make the interesting observation that GATA3 expression is initiated in both polar and apolar cells in a polarity-independent manner. Do apolar cells subsequently downregulate GATA3 levels as they become directed towards the inner cell mass lineage?
References
Gerri, C., McCarthy, A., Alanis-Lobato, G., Demtschenko, A., Bruneau, A., Loubersac, S., Fogarty, N.M.E., Hampshire, D., Elder, K., Snell, P., et al. (2020). Initiation of a conserved trophectoderm program in human, cow and mouse embryos. Nature.
Sasaki, H. (2015). Position- and polarity-dependent Hippo signaling regulates cell fates in preimplantation mouse embryos. Seminars in Cell & Developmental Biology 47–48, 1–8.
White, M.D., Zenker, J., Bissiere, S., and Plachta, N. (2018). Instructions for Assembling the Early Mammalian Embryo. Developmental Cell 45, 667–679.
Zhu, M., Leung, C.Y., Shahbazi, M.N., and Zernicka-Goetz, M. (2017). Actomyosin polarisation through PLC-PKC triggers symmetry breaking of the mouse embryo. Nature Communications 8, 1–16.
doi: https://doi.org/10.1242/prelights.25080
Read preprint










 (2 votes)
(2 votes)