Membrane architecture and adherens junctions contribute to strong Notch pathway activation
Posted on: 5 July 2021
Preprint posted on 26 May 2021
Article now published in Development at http://dx.doi.org/10.1242/dev.199831
These junctions are top notch: formation of Adherens Junctions enables Notch pathway activation during Drosophila cellularization.
Selected by Ilaria Di MeglioBackground
The Notch pathway is a highly-conserved pathway that mediates cell-to-cell communication throughout development, in tissue homeostasis and in disease. In normal conditions, interaction between transmembrane (TM) Notch receptor(s) and ligands of the Delta or Serrate/Jagged families cleaves Notch, releasing the Notch intracellular domain (NICD), which translocates to the nucleus to regulate transcription of target genes. Clearly, tissue geometry and the nature of cell contacts – their size, composition or dynamics – impact the level and duration of signals that cells receive from neighbours [1]. A better understanding of the contribution of tissue architecture to Notch signalling may thus provide further insight into how signalling is deployed in the different processes it controls. In this preprint, Bray and Falo-Sanjuan assess the role of cell architecture during cellularization in Drosophila, a morphogenetic process that involves profound morphological changes corresponding to the onset of Notch signalling.
Key findings
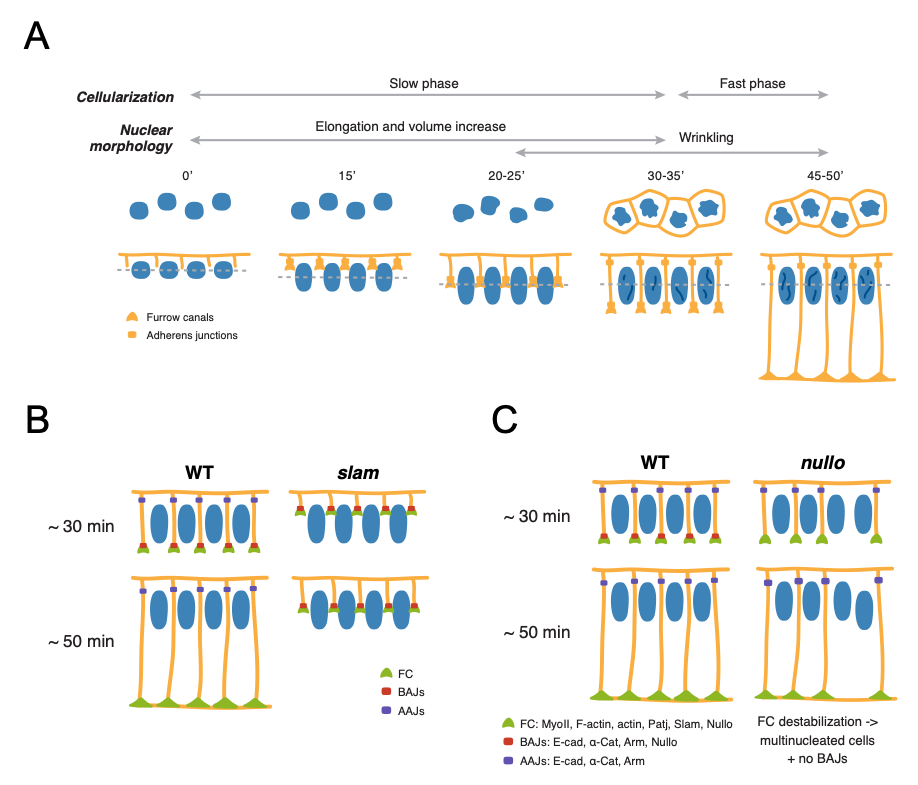
To assess the contributions of tissue architecture to Notch signalling, the authors focus on two processes, nuclear maturation and cellularization, during which profound tissue shape changes occur concurrently to Notch signalling initiation. The Drosophila embryo, initially a syncytium with nuclei that divide but are not separated by membranes, undergoes cellularization during nuclear cycle 14 (nc14), generating approx. 6000 cells with membranes surrounding each nucleus. During this stage, substantial changes in nuclear morphology also occur. Interestingly, activity of the Notch responsive enhancer m5/m8 is first detected at around 30-35 minutes into nc14, in contrast to other enhancers that are active throughout nc14, suggesting that nuclear maturation and cellularization may govern the onset of Notch-dependent transcription. The authors began by characterizing how each of these processes related to the timing of transcription.
To assess morphological changes occurring during nc14, Bray and Falo-Sanjuan used live imaging of embryos expressing Nup107-GFP and Spider-GFP to visualize nuclear shape changes and plasma membrane changes, respectively. During nc14 (Fig. 1A), nuclei elongate and increase in volume up to 30-35’ into the cycle, and subsequently undergo wrinkling of the nuclear envelope at the onset of signalling-dependent transcription. Cellularization is also characterized by two phases: an initial slow phase (30-35 minutes) where membranes grow and reach the inferior margin of nuclei, corresponding to the onset of transcription, and a fast phase (15 minutes) that concludes cellularization. To evaluate whether Notch signalling could be coupled to the observed morphological changes, the authors imaged embryos expressing Notch-GFP and D1-Scarlet and found that both Notch and Delta localized to the growing lateral membranes during the transition between the slow and fast phases of cellularization, when nuclear wrinkling also occurs. By monitoring m5/m8 dependent transcription, the authors found that these changes also correspond to the onset of transcription from m5/m8, suggesting that Notch signalling is initiated before the end of cellularization.
To uncover whether nuclear shape changes or membrane changes regulate Notch signalling, Bray and Falo-Sanjuan assessed a number of mutant embryos: embryos mutant for kugelkern, which encodes a nuclear Lamin protein, and embryos mutant for slam and nullo, encoding Slam and Nullo proteins which are required for furrow invagination during Drosophila cellularization [2]. While Notch-dependent transcription was unaffected in kuk mutants where nuclear elongation and wrinkling is disrupted, embryos exhibited almost no transcriptional activity in slam mutant embryos (Fig. 1B). Because both Slam and Nullo normally localize on the basal membrane of cells where they regulate actomyosin contractility, the lack of transcriptional activity in slam mutants indicates that lateral membranes contribute to Notch signalling. Interestingly, analysis of all mutants, including those exhibiting delayed rather than blocked cellularization – corresponding most probably to slam+/- heterozygotes – revealed that Delta expression correlates with delayed lateral membrane growth. These findings further confirm that Delta-Notch signalling depends on a specific step that occurs during and before the end of cellularization.
At this stage, the authors wondered whether signalling initiation depends on the growth of lateral membranes or formation of specific cell-cell junctions. To this end, Bray and Falo-Sanjuan analyzed transcriptional activity in embryos mutant for nullo, which do not properly form basal adherens junctions (BAJs) but exhibit normal lateral membrane growth. However, nullo mutants exhibited no correlation between membrane length and transcriptional onset time, and transcriptional levels resembled control embryos, suggesting that membrane length is not limiting initiation of signalling (Fig. 1C). On the other hand, disruption of both basal and apical AJs, assessed through knockdown of α-Catenin (α-Cat), had no effect on Notch distribution but did impair Notch-dependent transcription. Thus, formation of AJs, either directly or indirectly, contributes to Notch activation. Indeed, further experiments using fluorescence recovery after photobleaching (FRAP) used to assess Notch-GFP turnover revealed faster Notch recovery times upon α-Cat depletion at mid-cellularization compared to early time points, indicating that α-Cat contributes to stabilising Notch on the membrane.
Why I liked this preprint
I really enjoyed reading this preprint because it provides a simple but thorough mechanistic assessment of how cell-cell contacts may influence initiation of Notch signalling. It is becoming increasingly clear that mechanical cues, such as those arising from tissue architecture like cell-cell and cell-ECM interactions, provide an important contribution to morphogenetic processes throughout development. I therefore particularly liked the work by Julia Falo-Sanjuan and Sarah J. Bray, which focuses on the contribution of cell-cell adhesions to a crucial developmental pathway, Notch signalling.
Questions to authors
- Can you exclude that nuclear volume does not also contribute to Notch signalling? In kuk mutant embryos nuclei show reduced elongation and no wrinkling, but volume increases similarly to wild type.
- Could it be that mechanical forces provided by AJs (through adhesion between neighbouring cells as the lateral membranes grow) promote release of the Notch intracellular domain (NICD) in order to upregulate gene transcription? If so have you investigated this aspect further?
References
- Shaya, O., et al., Cell-Cell Contact Area Affects Notch Signaling and Notch-Dependent Patterning. Dev Cell, 2017. 40(5): p. 505-511 e6.
- Acharya, S., et al., Function and dynamics of slam in furrow formation in early Drosophila embryo. Dev Biol, 2014. 386(2): p. 371-84.
doi: https://doi.org/10.1242/prelights.29877
Read preprint










 (No Ratings Yet)
(No Ratings Yet)