Microbiota colonization tunes the antigen threshold of microbiota-specific T cell activation in the gut
Posted on: 14 October 2022
Preprint posted on 29 July 2022
The colonization status of the microbiota influences the antigen activation threshold of T-cells toward incoming bacteria in the gut.
Selected by Hervé BesançonBackground to the preprint
The gut microbiota is a very complex ecosystem, populated by lots of bacteria. In a healthy individual, the proportions of each bacterial species has reached a sort of equilibrium: species compete with each other for nutrients, but there are also symbiotic interactions that are established. Perturbations, like bad eating habits, can cause a species to outcompete the others, and a pathogen that disrupts the equilibrium can potentially cause health issues. Even if the problematic bug is eliminated, the microbiota can be in a shambles and have trouble reaching the previous healthy equilibrium again.
As awareness for the importance of the gut microbiota grows, so does the market for products that modulate the microbiota in order to improve human health. Products include supplements that introduce new commensal bacterial species or increase beneficial bacteria’s share of the microbiota. For such interventions to be efficient and safe, one needs to know how our body will react. Humans keep the bacteria from the gut contained, thanks to the epithelium, but the immune system remains informed of what’s going on through regular sampling. This then begs the question, what happens when we tinker with the gut microbiota?
In this preprint, Hoces et al. investigate what host conditions lead to the activation of T cells by Bacterioides thetaiomicron (B.theta), a commensal gut bacteria.
Key findings of the preprint
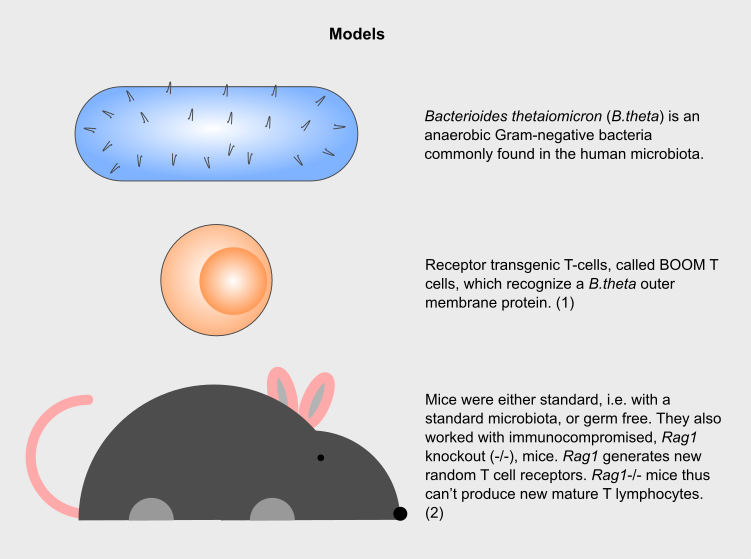
Figure 1: Models used in the study. Illustrations by Hervé Besançon
Mice with a different microbiota status (germ-free or standard) were colonized with B.theta and supplied with BOOM T cells. BOOM T cell activation and proliferation was tracked in the spleen and mesenteric lymph node (mLN, the membrane that connects the bowel to the abdomen).
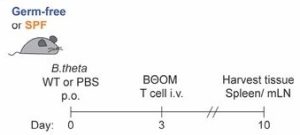
Figure 2: Main experimental design, taken from Figure 1 in the preprint by Hoces et al.
BOOM T cell proliferation is restricted in colonized mice
After introducing B.theta in germ-free mice, BOOM T cells were activated and then divided, both in the spleen and mLN. However, in standard mice, T cell activation and proliferation didn’t happen. The researchers investigated what could explain the dampened immune response in standard mice.
B.theta colonization in germ-free mice yielded a higher number of colony-forming units (CFU) in the cecum compared to standard mice, in which B.theta has to compete with the rest of the microbiota and is thus more restricted. Could this difference in bacterial load explain the difference in T cells activation?
In the mLN, CFU levels were similar in both groups suggesting a sampling independent of bacterial density. The authors tried to control for possible differences in antigen load between the two mice models by using a B.theta mutant lacking the outer membrane protein recognized by BOOM T cells. Using a 10:1 ratio in favor of KO B.theta, germ-free mice are still only colonized by B.theta bacteria but the BOOM antigen load is lower than in the first experiment. With that lower antigen load, a high percentage of BOOM T cells are activated, divide, but fail to survive, which suggests a threshold required for T cell maintenance.
Standard mice in which the microbiota was depleted by the use of antibiotics, allowing for a bigger B.theta population, also did not support BOOM T cell expansion. As antigen load is not the only parameter that influences T cell behavior, the researchers wondered whether prior colonization of the gut could affect the threshold for T cell activation.
Gut pre-colonization increased the threshold for BOOM T cell activation in immunocompetent mice
Germ-free mice were, during 6 days, colonized with a bacterial combination of the B.theta KO strain, commensal Eubacterium rectale and E.coli. The researchers then replaced the B.theta KO strain with the WT via targeted antibiotic treatment. Less BOOM T cells were activated in both spleen and mLN than in untreated germ-free mice. Prior bacterial exposure thus influences the T cell activation threshold for new bacteria.
In Rag1-/- mice, lacking a mature adaptive immune system and therefore also the competition between lymphocytes, the T cell reaction is the same whether or not the mice were previously colonized. No subset of the BOOM T cells became regulatory T cells (Tregs), whereas approximately 50% did in WT mice. Observing such a stark difference, the scientists explored what role Tregs could play in their model.
They used Treg-depleted mice. Without Tregs downregulation, BOOM T cells were activated irrespective of the B.theta strain that was used, which indicate antigen-independent activation. A sufficient Treg population and a competent mature immune system are required to dampen the immune response to new bacteria.
What I like about the preprint/why do I think it is important?
The gut microbiota is a very trendy research topic. Many companies try to ride this wave and use this attractive space to market loosely regulated products that aim to intervene, correct or balance this fragile ecosystem.
A lot of focus is placed on the microbial diversity or share of each bacterial species in the gut. Most studies do not look at what is happening in the surroundings of that ecosystem, including the host immune system that interacts with it. This research highlights the great complexity and diversity of factors at play to ensure the monitoring of the microbiota by the immune system.
- Given the difference between the two TCR-transgenic models tested, how generalizable do you think your observations are with regard to other antigens?
- How should external interventions on the microbiota evolve or what more should they take into account in light of the results presented in this paper?
- You showed that, in some cases, BOOM T cells proliferate but do not survive. In your opinion, why deploy so much resource and not preserve the cell?
(1) Wegorzewska MM, Glowacki RWP, Hsieh SA, Donermeyer DL, Hickey CA, Horvath SC, Martens EC, Stappenbeck TS, Allen PM. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Sci Immunol. 2019 Feb 8;4(32):eaau9079. doi: 10.1126/sciimmunol.aau9079. PMID: 30737355; PMCID: PMC6550999.
(2) Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869-77. doi: 10.1016/0092-8674(92)90030-g. PMID: 1547488.
doi: https://doi.org/10.1242/prelights.32913
Read preprint










 (No Ratings Yet)
(No Ratings Yet)