Microtubules tune mechanosensitive cell responses
Posted on: 13 September 2020
Preprint posted on 23 July 2020
Article now published in Nature Materials at http://dx.doi.org/10.1038/s41563-021-01108-x
Feeling filaments: A feedback loop between actin and microtubules regulates mechanosensing during cell migration
Selected by Grace LimBackground
To interact with their external environment, cells utilize a variety of protein complexes that connect them to neighbouring cells or to an underlying substrate. One such complex linking cells to the extracellular matrix (ECM) is the focal adhesion, comprising integrin receptors at the cell surface linked to actin filaments within the cytoplasm (Parsons et al., 2010). This integrin-actin link is mediated by a number of intermediary protein linkers, including talin and vinculin, which play critical roles in mechanical force transduction and activation of downstream signalling activities. As such, focal adhesions play a major role in transmitting mechanical information between the cell and its substrate to regulate adhesion and migration (Geiger et al., 2009).
Focal adhesions have primarily been studied in the context of their connection to actin filaments, but much less is understood about how microtubules (or intermediate filaments) could regulate and interact with focal adhesion complexes during migratory processes. In their new preprint, the authors begin to fill this gap by investigating the role of microtubule acetylation, a key post-translational modification, and uncover a crosstalk between actin and microtubules that regulate mechanosensitive cell migration.
Key findings
To understand the relationship between microtubule acetylation and focal adhesion-based mechanosensing pathways, the authors first explored the effect of altering substrate rigidity on acetylated tubulin levels. This revealed that cells grown on softer substrates displayed lower levels of acetylated tubulin than those on stiffer substrates, pointing to a mechanosensitive response by microtubules. Consistent with mechanosensing taking place at integrin-mediated focal adhesions, blocking integrin activity or inhibition of actomyosin contractility both led to reduced microtubule acetylation. These results point to mechanosensing via focal adhesions as a contributing factor in regulating microtubule acetylation levels.
The authors then focused on the microtubule acetylase aTAT1 as a key regulator of microtubule acetylation, which localizes specifically to focal adhesions in a microtubule-dependent manner. Importantly, mass spectrometry analysis to identify interacting partners revealed that aTAT1 binds talin, one of the mechanosensitive protein linkers within the focal adhesion complex. This aTAT1-talin interaction was reduced when actomyosin contractility was inhibited to block integrin signalling at focal adhesions. Together, these results suggest that mechanosensing via focal adhesions recruit aTAT1 to focal adhesions, where they promote microtubule acetylation.
What are the downstream consequences of microtubule acetylation at focal adhesion complexes? Using a number of elegant assays, the authors show that microtubule acetylation in fact go on to regulate RhoA activity (via localization of a specific RhoGEF) and cytoskeletal organization at focal adhesions. Microtubule acetylation by aTAT1 triggers the release of the active form of RhoGEF from microtubules, enabling the activation of RhoA-dependent actomyosin contractility. Moreover, microtubule acetylation also promotes greater traction force generation on the substrate, and is required for mechanosensing of substrate rigidity during collective migration. Indeed, this exemplifies a positive feedback loop whereby microtubule acetylation promotes Rho/actomyosin-dependent pathways to promote focal adhesion-based mechanosensing pathways, which in turn regulate the recruitment of aTAT1 to promote microtubule acetylation at focal adhesions. Loss of microtubule acetylation renders cells insensitive to substrate rigidity, underscoring the importance of microtubule acetylation in mechanosensitive cellular processes. In all, this preprint uncovers a novel mechanism whereby both actin filaments and microtubules work in concert to promote focal adhesion-dependent mechanosensitive cell migration.
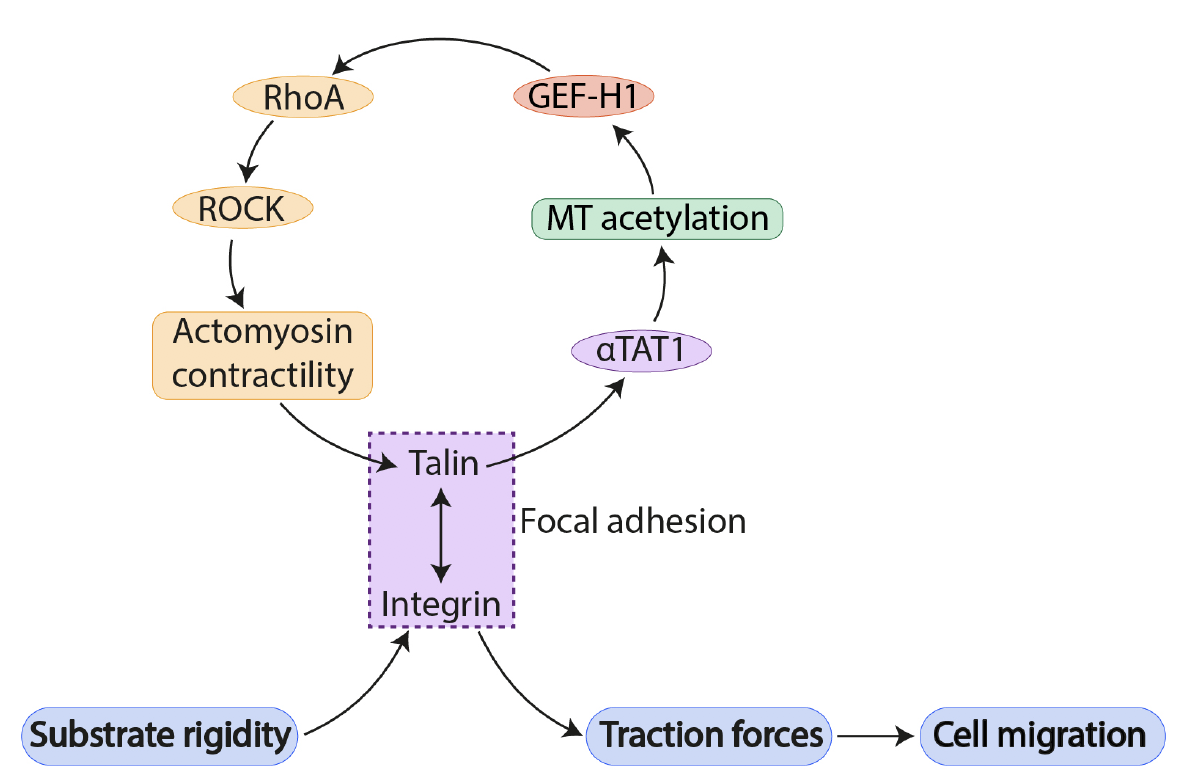
Figure 1. Feedback loop linking microtubule acetylation and actomyosin contractility in mechanosensing and cell migration at focal adhesions. Adapted from Figure 5D of Seetharaman et al. 2020.
What I like about this preprint
Despite the prevalence of cytoskeletal crosstalk between actin filaments, microtubules, and intermediate filaments (Chang and Goldman, 2004; Dogterom and Koenderink, 2018; Huber et al., 2015), these cytoskeletal proteins have largely been studied independently. The textbook model depicting focal adhesion complexes remains centred on actin filaments and actin-binding proteins that link to integrin receptors (Petit and Thiery, 2000). Recent work has started to uncover new roles for microtubules at focal adhesions, and how actin-microtubule interactions can regulate integrin signalling and cell migration. The findings from this new preprint thus add to this growing body of knowledge by identifying another key area of actin-microtubule crosstalk.
Future directions and questions for authors
Moving forward, it will be important to investigate the role of intermediate filaments in mechanosensing and cell migration, in relation to known actin- and microtubule-based pathways. Moreover, precisely how acetylation of microtubules influence downstream changes in RhoGEF localization remains to be elucidated. As the authors speculate, acetylation could alter microtubule conformation to induce detaching of RhoGEF from microtubules.
Questions:
- The authors report that aTAT1-depleted cells with reduced microtubule acetylation show alterations in cytoskeletal organization at focal adhesions, including thinner actin filament bundles, and reduced localization of microtubules and intermediate filaments. Can the authors speculate on the contribution of aTAT1 and/or microtubule acetylation to these cytoskeletal reorganization phenotypes? Are these changes independent of the Rho-ROCK pathway downstream of aTAT1?
- Apart from cell migration, focal adhesions are also important for cell adhesion and cell spreading, two processes that also involve mechanosensing of substrate rigidity. Can the authors comment on or speculate whether the microtubule acetylation-actomyosin feedback loop also takes place in these contexts?
References
- Chang, L., and Goldman, R.D. (2004). Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5, 601–613.
- Dogterom, M., and Koenderink, G.H. (2018). Actin–microtubule crosstalk in cell biology. Nature Reviews Molecular Cell Biology.
- Geiger, B., Spatz, J.P., and Bershadsky, A.D. (2009). Environmental sensing through focal adhesions. Nature Reviews Molecular Cell Biology 10, 21–33.
- Huber, F., Boire, A., López, M.P., and Koenderink, G.H. (2015). Cytoskeletal crosstalk: when three different personalities team up. Current Opinion in Cell Biology 32, 39–47.
- Parsons, J.T., Horwitz, A.R., and Schwartz, M.A. (2010). Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature Reviews Molecular Cell Biology 11, 633–643.
- Petit, V., and Thiery, J.-P. (2000). Focal adhesions: Structure and dynamics. Biology of the Cell 92, 477–494.
doi: https://doi.org/10.1242/prelights.24687
Read preprint










 (No Ratings Yet)
(No Ratings Yet)