Mitotic exit is controlled during anaphase by an Aurora B-Cyclin B1/Cdk1 crosstalk
Posted on: 29 April 2019 , updated on: 2 May 2019
Preprint posted on 12 April 2019
Article now published in eLife at http://dx.doi.org/10.7554/eLife.47646
The clock and the ruler: key kinases track anaphase in space and time to regulate the timing of mitotic exit
Selected by Dey LabNitya Ramkumar* and Gautam Dey*
*MRC Lab for Molecular Cell Biology, University College London
Background
Each cell cycle, eukaryotic cells must faithfully partition their genomes, organelles and cytoplasm into two daughter cells. Both entry and exit from this process of mitosis are tightly regulated by complex signalling networks that ensure fidelity and robustness. While mitotic entry has been studied in great detail for many years (1), the regulation of mitotic exit is much less well understood. The canonical “timer” or “clock” model posits that mitotic exit is triggered at anaphase onset, as soon as the spindle assembly checkpoint (SAC) is satisfied. Cyclin B1 is targeted for degradation by the anaphase-promoting complex (APC), leading to a reduction in Cdk1 activity (2). The dephosphorylation of Cdk1 substrates induces nuclear envelope reformation (NER) and DNA decondensation (3). In this model, Cyclin B1 degradation sets the clock- and the actual events of anaphase have no impact on the timing of mitotic exit.
Anaphase, however, is a complex multi-step process (4). Lagging chromosomes and DNA bridges can exhibit delayed decondensation and NER in an Aurora B-dependent fashion (5), providing evidence for a molecular “ruler” that is able to transduce local spatial information (chromosome location) into mitotic exit signalling. The authors here examine the possibility of crosstalk between the Cyclin B1-Cdk1-APC clock and the Aurora B ruler, as a mechanism to allow for error correction before the daughter cells enter the next cell cycle.
Key findings
Using Cyclin B1 and Aurora B as readouts, the authors demonstrate that the clock and ruler signalling pathways can influence each other.
While they do observe the (expected) dramatic reduction in Cyclin B1 levels at the metaphase-anaphase transition, they also find a residual pool of Cyclin B1 at centrosomes and the central spindle that continues to degrade throughout anaphase- and its degradation is essential for NER and DNA decondensation. They use multiple systems- human cell lines, Drosophila S2 cells and mouse oocytes- to demonstrate that this phenomenon of anaphase Cyclin B1 degradation is widely conserved. Further, using point mutations in the KEN-box domain (the domain recognized by APC/CCdh1) and acute inhibition of APC/CCdh1 activity, they show that the anaphase degradation of Cyclin B1 is regulated by APC/CCdh1.
The authors then go on to characterise the different pools of Cyclin B1 during anaphase. They show that the localisation of the mid-spindle pool (observed before in Drosophila germ cells (6)) depends on mKLP2 (Subito), a kinesin required for Aurora B re-localization to the midzone (7). Previous work has shown that Aurora B can phosphorylate Cyclin B1 (6); here, the authors demonstrate that a phospho-mimetic Cyclin B1 is more stable than wild type and thereby increases the duration of anaphase.
The authors conclude that the active crosstalk between the ruler and clock is mediated by Cyclin B1 and Aurora B during anaphase (Figure 1). The drop in Cdk1 activity at the metaphase to anaphase transition promotes the activation and re-localization of Aurora B to the central spindle and establishes a gradient of Aurora B activity (high in the middle and low towards the poles). This Aurora B gradient phosphorylates and stabilizes a pool of Cyclin B1, through which it controls the timing of mitotic exit.
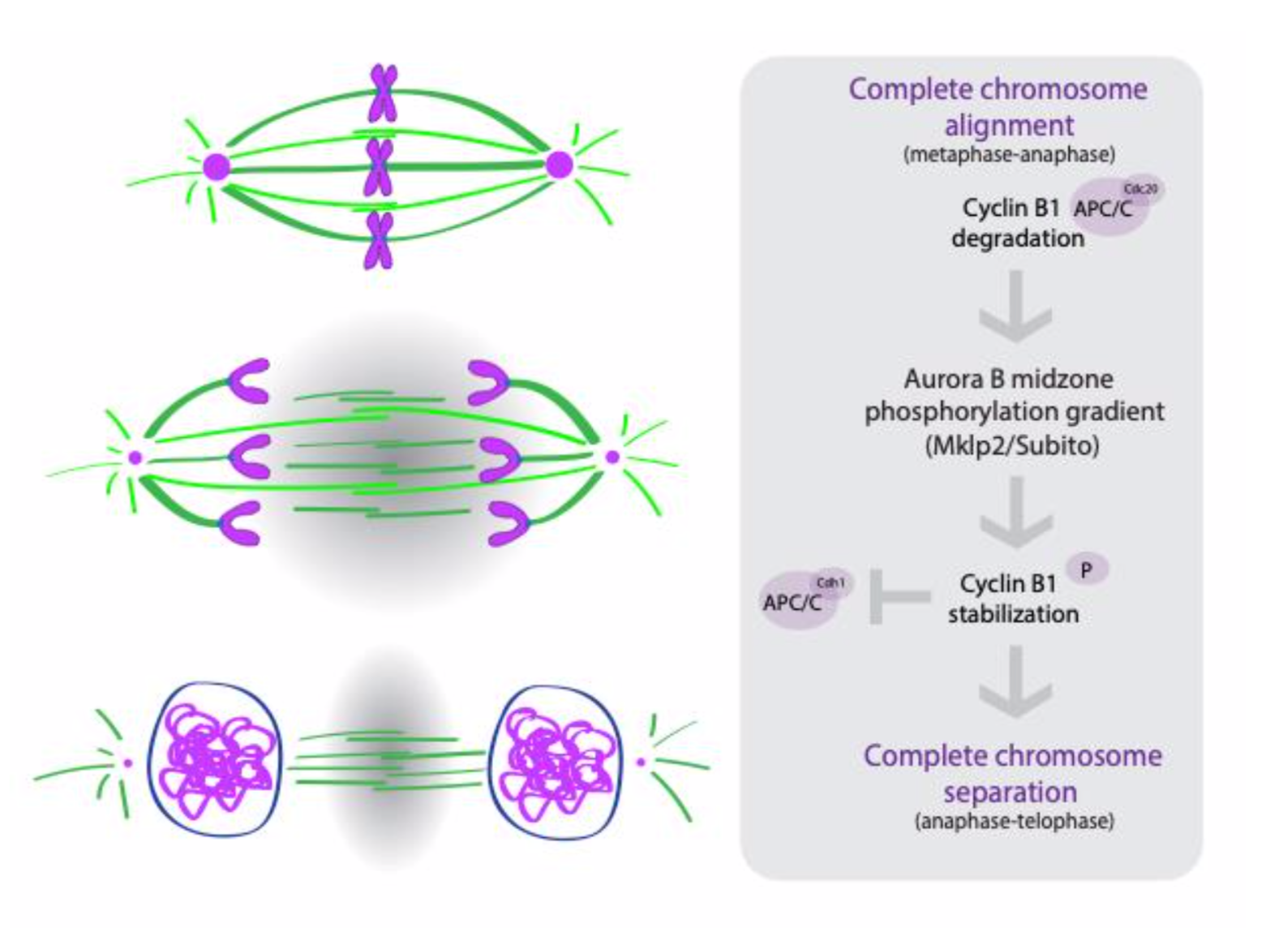
Why we chose this preprint
In this preprint, the authors neatly reconcile observations from multiple groups, proposing that two independent pathways can combine to correct segregation errors and control the duration of anaphase and mitotic exit. By using both Drosophila and human cell lines for their experiments, they also suggest that this regulatory architecture could be widely conserved.
The conventional view in the field holds that mitotic exit is regulated at the metaphase-anaphase transition, an irreversible event once the SAC has been satisfied. By shifting focus away from the SAC- and proposing that mitotic exit is actually determined during anaphase- this preprint opens up the field to further mechanistic analyses of the links between anaphase events, segregation errors, proteasomal regulation and entry into the next cell cycle.
Questions for the authors
- How do the levels of Cyclin B1 correspond to the duration of mitotic exit- is there a threshold below which the pathway is activated, or it is a continuous response?
- Are the different pools of Cyclin B1 differentially regulated? Which pool would be an ideal readout for mitotic exit duration?
- Does inhibition of Aurora B have an effect on the levels of Cyclin-B at the midzone?
- Do the Cyclin B1 phospho-mutants have altered localization dynamics, i.e. centrosomal vs the midzone?
- How are the levels of Cyclin B1 affected by DNA-bridges/lagging chromosomes, which can lead to prolonged Aurora B activation?
- How do the mitotic phosphatases feed into this regulatory circuit? Since the inhibition of phosphatases has been shown to affect NER, what is their mode of action if not through Cyclin B1 levels in the midzone?
References
- L. Pintard, V. Archambault, A unified view of spatio-temporal control of mitotic entry: Polo kinase as the key. Open Biol.8, 180114 (2018).
- A. Hershko, Mechanisms and regulation of the degradation of cyclin B. Philos. Trans. R. Soc. London. Ser. B Biol. Sci.354, 1571–1576 (1999).
- C. Wurzenberger, D. W. Gerlich, Phosphatases: providing safe passage through mitotic exit. Nat. Rev. Mol. Cell Biol.12, 469–482 (2011).
- N. Ramkumar, B. Baum, Coupling changes in cell shape to chromosome segregation. Nat. Rev. Mol. Cell Biol.17(2016), pp. 511–521.
- O. Afonso et al., Feedback control of chromosome separation by a midzone Aurora B gradient. Science. 332, 332–6 (2014).
- J. Mathieu et al., Aurora B and cyclin B have opposite effects on the timing of cytokinesis abscission in Drosophila germ cells and in vertebrate somatic cells. Dev. Cell. 26, 250–65 (2013).
- J. Landino et al., Two mechanisms coordinate the recruitment of the chromosomal passenger complex to the plane of cell division.Mol. Biol. Cell. 28, 3634–3646 (2017).
doi: https://doi.org/10.1242/prelights.10372
Read preprint










 (No Ratings Yet)
(No Ratings Yet)