Temporal changes in plasma membrane lipid content induce endocytosis to regulate developmental epithelial-to-mesenchymal transition
Posted on: 15 December 2020 , updated on: 17 December 2020
Preprint posted on 19 October 2020
Article now published in Proceedings of the National Academy of Sciences at http://dx.doi.org/10.1073/pnas.2212879119
Preparing to launch: changes in membrane lipid composition contribute to initiate epithelial-mesenchymal transition in neural crest cells
Selected by Glò Casas GimenoIntroduction
Epithelial-to-mesenchymal transition (EMT) is a paradigmatic cellular trans-differentiation process where epithelial cells acquire a mesenchymal migratory phenotype. This encompasses profound changes in gene expression and cell architecture, most characteristically, loss of apicobasal polarity and cell-cell adhesion, and assembly of actin stress fibers. EMT occurs in a variety of biological contexts: it is a well-studied phenomenon in cancer progression [1], but also a key step in wound healing, and in several developmental processes. Of the latter, the classic example is the neural crest EMT [2]. Neural crest cells originate from the neuroepithelium, and undergo a mesenchymal transformation that enables their delamination and migration to distant tissues, where they give rise to different cell types (e.g. smooth muscle, peripheric neurons, melanocytes).
In light of recent observations that lipid metabolism plays an active role in EMT in the context of cancer [3,4], Piacentino and colleagues set out to investigate if this might be the case also for neural crest EMT. Using transcriptomics data and considered in vivo manipulations in chicken embryos, they establish a model whereby ceramide production at the plasma membrane induces endocytosis of Wnt and BMP signaling components, which triggers downstream activation of the EMT transcriptional program.
I found this pre-print very enjoyable to read, especially because I was pleased to be reminded of the fact that the lipid plasma membrane is not an inert, passive holder of signaling proteins, but rather a dynamic structure with an active influence on signaling and cell behavior.
Research summary
To get some leads into the possible involvement of lipid metabolism in EMT, the authors compared transcriptomics data of chicken neural crest cells at pre-migratory vs. migratory stages and selected differentially expressed lipid-related genes. The authors chose to focus on the SMPD3 gene, which showed high enrichment in migratory stages. SMPD3 codes for neutral sphingomyelinase 2 (nSMase2), an enzyme that metabolizes sphingomyelin into ceramide at the plasma membrane.
In situ hybridization in chicken embryos shows Smpd3 expression can be detected only just prior to neural crest EMT, and this expression is sustained in subsequent stages. Ceramide, the metabolic product of nSMase2, is likewise present in neural crest cells.
Functionally, disruption of Smpd3 expression halts migration of neural crest cells, as the authors demonstrate both by electroporation of translation-blocking morpholinos (Figure) and CRISPR-mediated knock-out. Subsequent experiments reveal that nSMase2 knock-down precludes expression of transcription factors Sox9 and Snai2, which orchestrate neural crest EMT. At this point, the question is how does nSMase2, a ceramide-producing enzyme that acts at the membrane, regulate gene transcription.
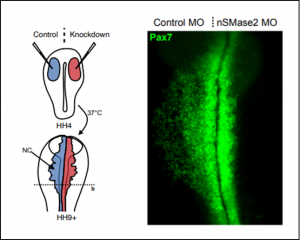
Previous research in the fly wing disc discovered a role for ceramide synthases in strengthening Wnt signaling by facilitating endocytosis of its components [5]. As it happens, Wnt signaling is necessary to initiate the neural crest EMT transcriptional program and directly regulates Snai2 expression, which led the authors to hypothesize that nSMase2 might promote neural crest EMT through its role in Wnt signal transduction. Here, the authors extend this hypothesis to BMP signaling, as it is also a well-known upstream regulator of EMT, and both Wnt and BMP pathways partly rely on endocytosis.
In support of this hypothesis, the authors find that nSMase2 knock-down neural crest cells express lower levels of Wnt and BMP fluorescent reporters. Following this line of evidence, blocking endocytosis by expression of a dominant negative Dyn1(K44A) mutant specifically in the neural crest recapitulates nSMase2 knock-down phenotypes: arrested neural crest migration and reduced Wnt signaling activity.
Importantly, the authors directly tested the role of nSMase2 in endocytosis in an in vitro Transferrin internalization assay. Very encouragingly, over-expression of nSMase2 in U2OS cells results in a significant increase in Transferrin internalization, an effect that is dependent on the functional catalytic activity of nSMase2.
Questions for the authors
- Do you have any hypotheses on the possible role of other genes in your list of lipid-related differentially expressed genes, and did you/will you investigate them further?
- As you note, the in situ data show nSMase2 expression also in the neural tube and notochord. Did you detect any obvious defects in these structures following nSMase2 knock-down?
- In the experiment where you block endocytosis by expression of a dominant negative form of Dyn1, you show the effects this has on Wnt reporter expression and neural crest migration. Did you also check for changes in BMP reporter expression, or changes in EMT transcriptional regulators Msx1, Sox9 or Snai2?
- Did you consider attempting to visualize in vivo the endocytosis of Wnt or BMP signaling components in nSMase2 morphant vs. control?
- According to the model that ceramide synthesis favors endocytosis of Wnt and BMP signaling components in the neural crest, do you think that modulating ceramide membrane composition through an alternative mechanism would result similar phenotype?
References
[1] Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019.
[2] Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994
[3]. Zheng K, Chen Z, Feng H, Chen Y, Zhang C, Yu J, Luo Y, Zhao L, Jiang X, Shi F. Sphingomyelin synthase 2 promotes an aggressive breast cancer phenotype by disrupting the homoeostasis of ceramide and sphingomyelin. Cell Death Dis. 2019
[4] Wang W, Hind T, Lam BWS, Herr DR. Sphingosine 1-phosphate signaling induces SNAI2 expression to promote cell invasion in breast cancer cells. FASEB J. 2019
[5] Pepperl J, Reim G, Lüthi U, Kaech A, Hausmann G, Basler K. Sphingolipid depletion impairs endocytic traffic and inhibits Wingless signaling. Mech Dev. 2013
doi: https://doi.org/10.1242/prelights.26404
Read preprint










 (1 votes)
(1 votes)