Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain
Posted on: 15 May 2020
Preprint posted on 13 February 2020
Article now published in Neuron at http://dx.doi.org/10.1016/j.neuron.2021.11.020
Macrophages in the spotlight: resolving chronic pain by extracellular-vesicle mediated transfer of mitochondria to sensory neurons.
Selected by Giuliana ClementeContext and Background:
Inflammation is the physiological response of body tissues to mechanical stress and infection and is characterized by the rapid infiltration of immune cells to the site of damage and the local release of a variety of molecular mediators which are beneficial to proper repair and healing. The release of these mediators is often associated with the stimulation of nociceptors and evokes a sharp feeling of pain which protects the site of injury from further damage. Normally, inflammatory pain fades away once the tissue is properly healed. However, there are patients who develop the so-called chronic pain syndrome (CPS), whereby the pain persists far beyond the normal time of recovery becoming in fact chronic. This condition affects 8% of young people between the age of 13 and 18 and 10% of the adult population and represents a serious public health issue that greatly reduces everyday quality of life.
Although the mechanism that leads to the development of inflammatory pain is overall well understood, how pain is resolved is way less characterized and gaining a better understanding of this process will eventually lead to the development of more effective treatments for CPS.
The preprint in pills:
The preprint highlights a previously uncharacterized role for macrophages in actively promoting the resolution of inflammatory pain and hyperalgesia by transferring mitochondria to sensory neurons through the release of extracellular vesicles (EVs). Authors provide molecular insight into this process, showing how transfer of EVs between these cell types requires the interaction between the CD200 receptor on the surface of macrophages and the non-canonical ligand iSec1 on the surface of sensory neurons (Figure 1).
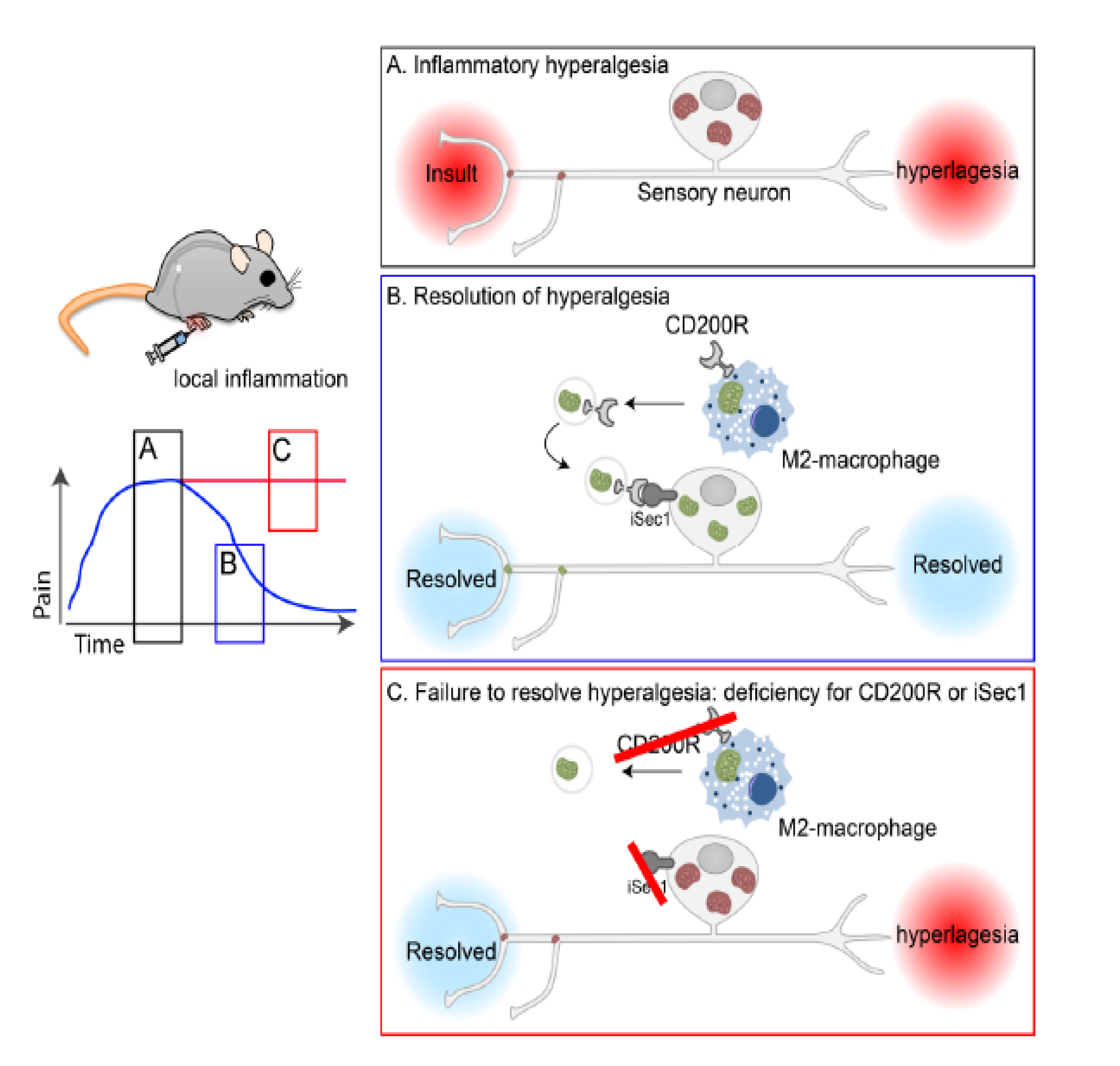
Figure 1: Graphical abstract adopted from the preprint.
M2 macrophages promote the resolution of inflammatory hyperalgesia through the release and transfer of EVs loaded with fully functional mitochondria to sensory neurons. The transfer of mitochondria between these two cell types relies on the interaction of the receptor CD200R with the non-canonical ligand iSec. Through this mechanism, macrophages help neurons to replenish their pool of mitochondria and contribute to the resolution of inflammatory pain.
Key points:
In this preprint the authors investigated the role of the immune system in the resolution of inflammatory pain using carrageenan-injected mice as working model. Injection of carrageenan in the hind paw triggered an inflammatory response characterized by marked infiltration of macrophages. Macrophage number reached a peak within 3 days post-treatment to then return to baseline levels in concomitance with the resolution of inflammatory pain. Furthermore, MMdtr mice depleted of monocytes and macrophages, mounted a robust, long-lasting hyperalgesia which was solved only upon bone marrow transplantation, reinforcing the idea that localized activity of macrophages was crucial to resolving inflammatory pain.
Given that neurons face a high metabolic demand to perform their function and that mitochondrial disfunction in neurons is associated with CPS, is it possible that infiltrated macrophages alleviate inflammatory pain by helping sensory neurons in restoring a functional pool of mitochondria? Transferring of mitochondria between cell types could be visualized both in vitro and in vivo by labelling macrophage mitochondria using fluorescent probes. In addition, genetically removing either mitochondria or macrophages, significantly reduced the number of neurons bearing macrophage-derived mitochondria, validating the hypothesis that transfer of mitochondria between these cell types contribute to the resolution of pain.
How does this transfer take place? Flow cytometry-based analysis revealed that macrophages released extracellular vesicles (EVs) of different sizes which stained positive for mitochondria-fluorescent dyes, suggesting they carry mitochondria. In addition, injection of mitochondria-bearing vesicles was sufficient to solve inflammatory pain. Finally, the authors uncovered the basic molecular machinery required for the docking of the vesicles on the surface of the sensory neurons. Transferring of the vesicle content relied on the expression of the CD200 receptor (CD200R) on the surface of macrophages (and therefore of the EVs budding off from their surface) and the expression of the non-canonical CD200R ligand, iSec1 on the surface of sensory neurons. In fact, either knockout of CD200R or RNAi-mediated downregulation of iSec1 in neurons significantly compromised mitochondria transfer and therefore the resolution of inflammatory pain. Finally, expression of an RNAi-resistant iSec in sensory neurons of iSec1-RNAi, CD200-null mice completely restored the ability of these animals to solve pain.
Relevance:
The data presented in this preprint uncover in vivo a novel role for macrophages in the resolution of inflammatory pain which is achieved by transferring mitochondria to sensory neurons via EV release. This work has important implications for the development of novel therapies to resolve inflammatory pain which might either aim to restore the functional pool of mitochondria or boosting the transferring of mitochondria from macrophages.
Questions to the authors:
- Do you know whether macrophages transfer other mediators to sensory neurons via extracellular vesicles to solve inflammation and pain? Are you planning to perform a detailed profiling of the content of these vesicles?
- Is there an optimal number of mitochondria that get transferred to allow resolution of pain? About the size of the vesicles: have you observed any correlation between size of the vesicles and content/number of mitochondria transferred?
- Can you speculate on mechanism(s) that allow macrophages to cope with the loss of mitochondria after the EV-mediated transfer to neurons? Is de novo biogenesis of mitochondria taking place?
- Is there any GWAS study which correlates the development of hyperalgesia to SNPs on the CD200R gene or its ligands in patients with CPS?
- Would you be able to explore whether mechanism similar to the one reported in this study take place in animal model of epilepsy?
- Where do you think the field will move? Can you exploit this discovery (release of macrophage derived EVs) and harness macrophages to carry and transfer specific cargos?
doi: https://doi.org/10.1242/prelights.20690
Read preprint










 (No Ratings Yet)
(No Ratings Yet)