Jonathon Muncie (J.M.M.), Nadia Ayad (N.A.) and Johnathon Lakins (J.N.L.) shared
- Why do you see multiple streak-like structures (high T+) in the unrestricted conditions? Is this a function of density?
“We think that the formation of multiple discrete T-positive “streaks” or “nodes” may be a combination of both non-confinement and preparation of embryonic stem cell colonies on soft(er) substrates.
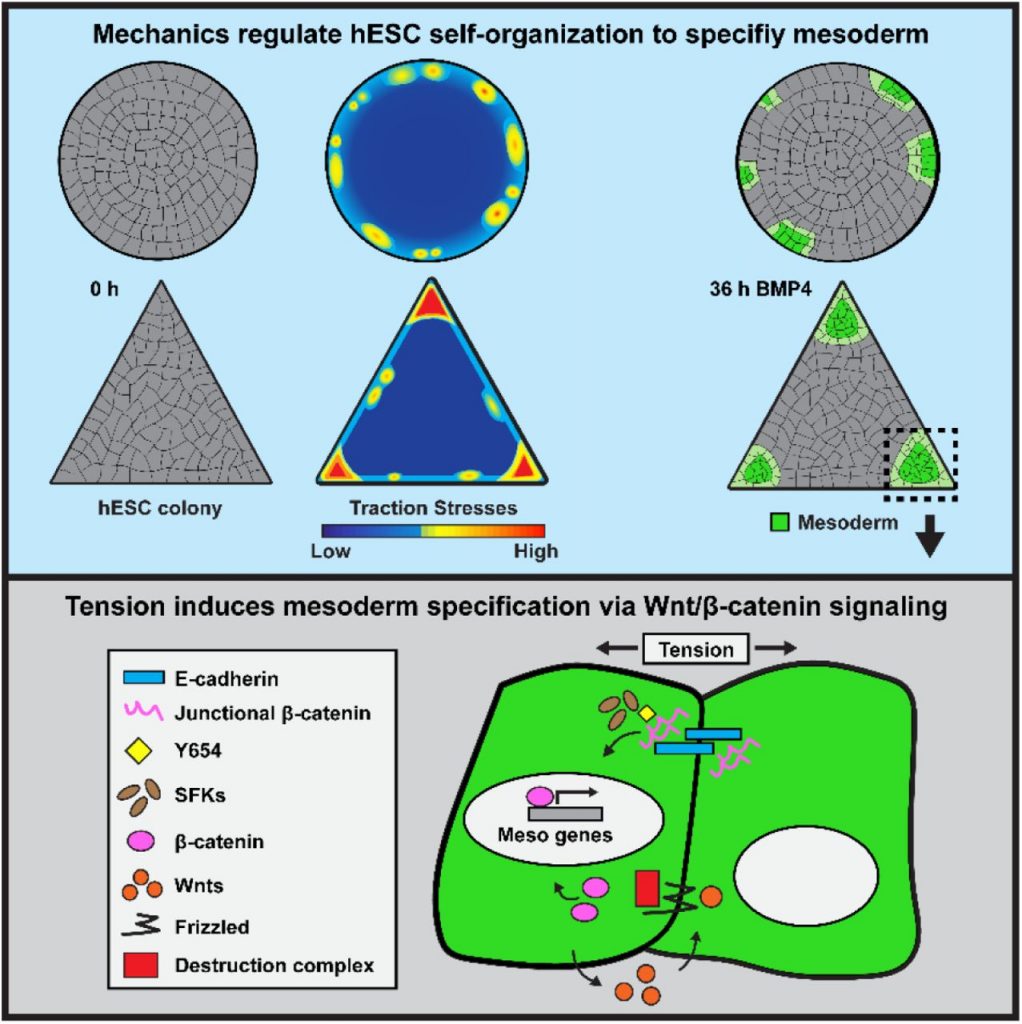
To digress a bit, by way of explanation, in our previous work we showed that hESC colonies on unconfined softer substrates promoted a more robust mesoderm differentiation response than stiff substrates [1]. This was attributed to a more mechanically permissive environment for epithelialization, i.e. cells readily acquired a denser, more columnar morphology, in part attributable at the molecular level to formation of E-cadherin- and β-catenin-rich adherens junctions (AJ). In contrast, hESCs on stiff substrates experienced inhibitory effects due to β1-integrin-driven cell spreading and reduced AJ formation.
To simplify our most recent results, the amount and/or state (i.e. stretched vs. relaxed) of AJ associated β-catenin acts as a downstream (to BMP4/SMAD signaling) “gain” function in mesodermal commitment, seemingly thresholding commitment and/or altering the kinetics. In unconfined colonies on stiff substrates marginal (edge) cells simply keep expanding outwards with a flattened aspect and are not able to efficiently re-assemble AJ, and thus lack this gain function β-catenin signaling. When confined, even on stiff substrates, as in previous work from other labs, crowding may enhance epithelialization, hence edge cells are susceptible to mesoderm commitment.
The question is whether all edge cells are equally susceptible to mesoderm commitment, i.e. whether all edge cells are capable of becoming T-positive upon BMP4 treatment. In circular confined colonies on stiff substrates, to the extent that it has been studied, the answer appears to be a qualified yes. As we have shown in this work, in confined colonies of non-circular geometries, although all edge cells seem to be susceptible to mesoderm commitment, some become T-positive more readily and rapidly than others (vertex of triangle vs. straight edge). In undifferentiated circular colonies on unconfined (and confined) soft substrates the colony exerts an inward radial tension on the substrate born most strongly by edge cells. However, this inward tension is not equal everywhere around the circumference but tends to exhibit discrete nodes of higher and lower tension around the edge repeating with some seeming regularity. The origin of this discretization is unknown, although a possible morphomechanical mechanism can be found in [2]. However it is formed, we believe it guides in some fashion the subsequent formation of T-positive nodes. We also speculate that in some way this discretization may arise more readily or even be permissible only on softer substrates in unconfined colonies. A more detailed dissection of the dynamics of matrix tensions both before and after differentiation is an active area of investigation in the Weaver Lab.
The other speculative, though likely contributing, factor in discretization of T-positive nodes is permissible patterns of cellular migration. In unconfined colonies, outward radial migration of edge cells is permitted upon BMP4 differentiation. At early time points, before edge cells have undergone a clear EMT and separated from the epithelial sheet, this migration appears to occur collectively as an epithelial sheet. Leading cells may pull on trailing cells to drive rear-ward convergence-extension of T-positive trailing cells into discrete radially elongated “nodes”. In confined colonies, the forced inward migration of T-positive cells either obscures or outright prevents the appearance of such distinct nodes.” – JNL
- In circular micropatterned stem cell colonies, the edge restriction of BMP signaling activity has been linked to differential receptor localization and the action of diffusible inhibitors (Tewary, et al., 2017; Etoc, et al., 2016).
- How do you reconcile reaction-diffusion models of spatial patterning and tension dependent patterning? To what extent do you think these mechanisms influence one-another, and do they initiate or propagate patterning?
“It’s clear that the reaction-diffusion kinetics of key morphogens and inhibitors are important for spatial patterning in early development. Likewise, in our system, tension alone is not sufficient for mesoderm specification, we must add BMP4 to induce differentiation, demonstrating a role for reaction-diffusion. However, whereas the previous studies you mentioned have used reaction-diffusion models to explain ring patterns of mesoderm specification that arise when hESC colonies are stimulated with BMP4 on hard substrates like tissue culture plastic or glass, when we perform similar experiments on soft hydrogels, we observe discrete nodes of mesoderm specification that coincide with regions of high tension, rather than continuous rings of mesoderm. We think that in the context of a soft microenvironment, the tension-induced Wnt/β-catenin signaling mechanism that we highlight is “layered” on top of classic reaction-diffusion models, leading to discrete nodes that receive the highest levels of mesoderm induction signals. We are currently working on designing experiments to test the relative importance of each mechanism by inducing tension in regions of colonies that reaction-diffusion models would suggest experience high concentrations of secreted inhibitors that should otherwise prevent mesoderm specification. Hopefully we will have more data to present in this area soon!” – JMM
- Do you have any insights into the relationship between tension and BMP?
Our lab previously showed that E-cad-based junction stabilization primes cells to receive mesoderm signals [1]. BMP signaling seems to have an instructive role in downregulating cadherin-adhesiveness through BMPR type Ia (BRIa) and increasing the stability of lamellipodia [3]. In addition, recent evidence argued that VE-cadherin physically interacts with BRIa and BMPR type II (BRIa and BRII), stabilizing complex formation [4]. Given the established role of E-cad as a force sensor [5] and research showing that TGF-β receptors, which share a high degree of homology with BMPRs, are sensitive to cellular tension, and form complexes at focal adhesions with integrin and the actin-binding protein cofilin [6], it is possible that BMPR trafficking and signaling might also be controlled by cell tension, through perhaps an interaction with E-cadherin or other mechanosensitive proteins.” – NA
- What role do you think force plays in the embryo, and how are extraembryonic structures contributing to this?
“The role of mechanical forces in embryogenesis is a broad and open-ended question. There is little dispute that mechanical forces are employed to sculpt the embryo through cellular migrations and topological transformations of epithelial sheets. In as much as these transformations might juxtapose different cells or alter the geometries of cellular communication, they can be said to play indirect permissive roles in the morphogenic signaling pathways that drive fate specification.
It is not quite as clear, or at least less appreciated, how mechanical forces could more directly influence internal cell signaling processes that underlies developmental decisions. Here and in our previous work using hESCs on soft matrices we have tried to show that mechanical forces may have a more direct effect on cell signaling processes that drive early mesodermal (T-positive) specification. In our earlier work [1], we made the important discovery that matrix stiffness affects epithelialization of hESC colonies, rendering them competent for mesoderm specification. This is important as it emphasizes that epithelialization is not a secondary or accompanying phenomenon to gastrulation, rather, it is intimately linked to it. Now, in the current work we have suggested that tension may directly drive a molecular state (stretched β-catenin at AJ) that was previously shown in lower organisms [7], in addition to increased levels of AJ associated β-catenin that we showed previously, to permit enhanced mesoderm specification.
We note that in the avian pre-gastrulation embryo, epithelialization of the blastoderm has been shown to proceed from the posterior margin (the domain of T expression and site of primitive streak formation; [8]) and a tension decaying from posterior to anterior has been shown to develop along the margin of the area pellucida [9]. Hence, it is possible that in the avian embryo the posterior margin has the highest (mechanically driven) competency for T induction and mesoderm specification. Thus, in addition to other regulatory mechanisms such as posterior Vg1 expression and the role of the hypoblast in suppressing ectopic streak formation, tension may play a reinforcing role in setting the domain of T expression and site of primitive streak formation.
Additionally, there may be a role for tension developed on the embryo proper by extra-embryonic cells. Referring again to the avian embryo, the cells of the extra-embryonic area opaca are in physical contact and connected to the embryonic area pellucida and are physically adhered at their edge to the overlying vitelline membrane. Radial expansion of area opaca edge cells over the vitelline membrane may transmit forces to the embryo, which could potentially influence some of the events described above.” -JNL
- Would it be possible to mimic the asymmetric structure of the PS in the embryo on a micropatterned substrate, expanding from the center to the edge of the colony by altering tension?
“Research has shown the importance of extraembryonic tissue [10] and stiffness [11] for morphogenesis during development, thus the substrate in which cells are growing, as we briefly show here, seems to be paramount to model development in vitro. Additionally, the role of controlled gradients of morphogens seems to be an important factor in providing the cues for symmetry breaking to occur [12]. It would be interesting to think of an experiment that would bring together both the micropatterning on mechanically tuned substrates that we present here plus microfluidics [13] to create a controllable gradient of morphogen and assess whether that would be able to provide a more accurate model of the asymmetric structure of the primitive streak.” – NA
- Is the increased release of β-catenin from cell junctions at high tension regions associated with an elevated WNT signaling response?
“So far, all the data we have indicates the answer is yes. Using ISH-HCR, observed that the key mesoderm-inducing Wnt, Wnt3a, was specifically transcribed in regions of high tension following the release of β-catenin. More importantly, when we use Src-family kinases inhibitors to prevent the release of β-catenin from cell junctions in high-tension regions, we observe a significant reduction of Wnt3a in high-tension regions, both by ISH-HCR and qPCR. This indicates that Wnt signaling, and Wnt3a levels in particular, are elevated by the release of β-catenin from cell junctions. Moreover, this elevated Wnt signaling seems to be critical for the level of mesoderm specification we observe. We have new data that shows inhibition of porcupine family proteins, which process Wnt ligands to mediate their secretion, significantly reduces mesoderm specification. Thus, both the initial release of β-catenin from cell junctions and the subsequent Wnt signaling are critical for mesoderm specification in regions of high tension.” – JMM
- What is the most important message that you would like readers to take home from your paper?
“In this paper we highlight how mechanical cues, namely tension, can feed into classical signaling pathways such as Wnt/β-catenin to tune the cellular response to morphogen cues. I hope readers will consider how tension and other physical forces may play a role in altering or tuning the signaling pathways and cellular responses in whatever context they may be studying.” – JMM
- What is your lab tradition for celebrating an accepted paper? Did you have a pre-print celebration?
Our lab meetings are typically held on Friday mornings, so we usually celebrate an accepted paper with champagne and mimosas at lab meeting. We typically would celebrate an initial submission or pre-print upload with a lab happy hour, but the COVID-19 pandemic kept us from doing that this time…or at least delayed us.
References
[1] L. Przybyla, J. N. Lakins, and V. M. Weaver, “Tissue Mechanics Orchestrate Wnt-Dependent Human Embryonic Stem Cell Differentiation,” Cell Stem Cell, vol. 19, no. 4, pp. 462–475, 2016.
[2] Beloussov, Lev V. 2008. “Mechanically Based Generative Laws of Morphogenesis.” Physical Biology 5(1): 15009. https://iopscience.iop.org/article/10.1088/1478-3975/5/1/015009/pdf (January 23, 2020).
[3] S. von der Hardt et al., “The Bmp Gradient of the Zebrafish Gastrula Guides Migrating Lateral Cells by Regulating Cell-Cell Adhesion,” Curr. Biol., vol. 17, no. 6, pp. 475–487, 2007.
[4] A. Benn, C. Bredow, I. Casanova, S. Vukičević, and P. Knaus, “VE-cadherin facilitates BMP-induced endothelial cell permeability and signaling,” J. Cell Sci., vol. 129, no. 1, pp. 206–218, 2016.
[5] N. Desprat, W. Supatto, P. A. Pouille, E. Beaurepaire, and E. Farge, “Tissue Deformation Modulates Twist Expression to Determine Anterior Midgut Differentiation in Drosophila Embryos,” Dev. Cell, vol. 15, no. 3, pp. 470–477, 2008.
[6] J. P. Rys et al., “Discrete spatial organization of TGFβ receptors couples receptor multimerization and signaling to cellular tension,” Elife, vol. 4, pp. 1–20, 2015.
[7] Brunet, T., Bouclet, A., Ahmadi, P., Mitrossilis, D., Driquez, B., Brunet, A.C., Henry, L., Serman, F., Bealle, G., Menager, C., et al. (2013). Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat Commun 4, 2821.
[8] Lee, Hyung Chul et al. 2020. “Molecular Anatomy of the Pre-Primitive-Streak Chick Embryo.” Open Biology 10(2): 190299. http://www.ncbi.nlm.nih.gov/pubmed/32102607 (March 31, 2020).
[9] Saadaoui, Mehdi et al. 2020. “A Tensile Ring Drives Tissue Flows to Shape the Gastrulating Amniote Embryo.” Science 367(6476): 453–58. http://science.sciencemag.org/ (January 27, 2020).
[10] Christodoulou et al., “Morphogenesis of extra-embryonic tissues directs the remodelling of the mouse embryo at implantation,” Nature Communications, 10, 2019
[11] Barriga et al., “Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo,” Nature, 2014
[12] Simunovice et al., “A 3D Model of a Human Epiblast Reveals BMP4-driven Symmetry Breaking ,” Nat. Cell. Bio., 2019
[13] Zheng et al., “Controlled modelling of human epiblast and amnion development using stem cells,” Nature, 2019











 (No Ratings Yet)
(No Ratings Yet)