Polarized endosome dynamics engage cytosolic Par-3 and dynein during asymmetric division
Posted on: 29 May 2020
Preprint posted on 16 May 2020
Article now published in Science Advances at http://dx.doi.org/10.1126/sciadv.abg1244
Removing the Notch: a coordinated effort of Par-3 and Dynein drives the asymmetric segregation of Delta-enriched endosomes.
Selected by Giuliana ClementeContext and Background:
Asymmetric cell division (ACD) is essential for the development of multicellular organisms and is a strategy utilized to generate cell diversity. This specialized mode of cell division is characteristic of stem cells which, at each cell cycle, divide to self-renew and to generate a daughter fully committed to differentiation. To allow ACD, cells establish apical-basal polarity axis through the assembly of cortical domains which ultimately facilitate the differential distribution of fate determinants at each division. Neurogenesis is extensively used as a model to explore mechanism(s) of ACD in many organisms and to uncover signaling pathways involved in the generation of fate diversity. A growing body of evidence in Drosophila as well as vertebrate models, point to a function for Notch in promoting cell fate switch upon ACD, with the cell receiving low Notch activity committed to differentiation while the one maintaining high level of Notch which keeps traits of stemness. However, how polarity proteins control segregation of Notch signaling components and where the interaction between polarity proteins (Par) and Notch take place is still unclear. In this preprint, the authors explore these questions using the zebrafish radial glia progenitors (RGPs) as working model.
Main findings:
The authors investigated how the polarity protein Par-3 contributes to the asymmetric distribution of Notch signaling in RGP cells. To study how this asymmetry arose, they performed an antibody uptake assay to visualize internalization and distribution of the Notch ligand Delta (Dld) during ACD (Figure 1). This in vivo analysis revealed that Dld is internalized in endosomes (Dld endosomes) which firstly converged towards the metaphase plate and afterwards towards the cleavage furrow during anaphase. At telophase, the Notch signaling endosomes are transported towards the posterior daughter cell where they accumulated. This movement of Notch endosomes to a more posterior location relied on the coordinated activity of the minus-end directed motor Dynein and a transient cytosolic pool of Par-3. This small pool of cytosolic Par-3 started to be detectable in the posterior daughter cell at anaphase and co-localized with Dynein in close proximity of Notch endosomes, as shown by label-retention expansion microscopy. In addition, removal of Par-3 caused a significant decreased in the co-localization of Dynein to Notch endosomes, suggesting that the polarity protein works as a scaffold that connects the Dld endosomes to the motor protein. This sophisticated strategy resulted in the accumulation of Dld endosomes and Notch receptors in the posterior daughter cell which, as a result of the activation of the Notch pathway, would eventually differentiate in neurons (Figure 1).
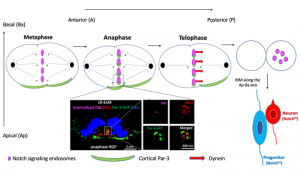
Figure 1: Schematic representation summarizing the main findings of the study. Endosomes enriched in the Notch ligand Delta and its receptor (in magenta) congregate to the cleavage furrow during metaphase and anaphase and move towards the posterior daughter cell at telophase in a Par-3 (in green) and Dynein (red arrow) dependent fashion. Co-localization of cytosolic Par-3 and Dynein at Dld endosomes could be visualized by label-retention expansion microscopy (image adopted from the preprint). The posterior daughter cell enriched in Dld endosomes move towards a more basal position upon completion of ACD, in a process known as Interkinetic Nuclear Migration (INM). Ultimately, high levels of Notch activation drive differentiation.
Relevance:
The work here highlighted uncovers a novel function for Par-3 in the asymmetric segregation of Notch signaling during RGPs division. Particularly, the work describes a novel mechanism whereby cytosolic Par-3 is directly involved in the segregation of Dld endosomes through the recruitment of Dynein. The work demonstrates that cytosolic Par-3 (and not only the cortical pool) plays an important role in the asymmetric segregation of cytosolic determinants.
Questions to the authors:
- Given the pleiotropic functions of Dynein in cells (and during cell cycle), do you think that your ciliobrevin treatment or the dlic morpholino are actually compromising Dld endosome internalization?
- How do you think this cytosolic pool of Par-3 is generated? Do you think cells express an isoform of Par-3 that lack the domain required for cortical localization? Or does this pool generate as a consequence of the saturation of the cortical domain?
- Given that the pool of cytosolic Par-3 is generated in a timely fashion (anaphase), how do you think the appearance of this pool is coordinated with cell cycle progression?
- Have you tested the direct interaction between Par-3 and Dynein to prove that Par-3 is actually the physical bridge that link endosomes to the Dynein motor?
doi: https://doi.org/10.1242/prelights.21360
Read preprint










 (1 votes)
(1 votes)