Social foraging in vampire bats is predicted by long-term cooperative relationships
Posted on: 28 May 2021
Preprint posted on 23 April 2021
Article now published in PLOS Biology at http://dx.doi.org/10.1371/journal.pbio.3001366
Blood sisters: vampire bats leave the roost alone but meet up with preferred roostmates to forage.
Selected by Lucy NevardCategories: animal behavior and cognition
Context
Access to food is a crucial benefit of cooperative relationships in group-living animals, ranging from birds to chimpanzees. Over the last few decades, the common vampire bat (Desmodus rotundus) has become a classic example of cooperation and reciprocal food-sharing – bats who fail to feed on a given night are provided for by other bats via regurgitation of blood meals. This food-sharing is based on social bonds formed through grooming in the roost and is not always driven by relatedness. Although we have detailed evidence of interactions and bond formation between roostmates, less is known about the impact of these relationships on activity outside the roost, i.e. bats’ nightly foraging behaviour. The authors of this work aimed to distinguish between six competing hypotheses regarding vampire bat foraging behaviour (Figure 1). They include the possibilities that socially bonded roostmates either prefer to or avoid feeding together, or that prior social association has no effect on foraging encounters.
A historical lack of data on foraging encounters is partly for practical reasons: it is technically challenging to track individual free-ranging bats. The authors chose to use custom-made proximity sensors, allowing them to continuously track 50 bats over several nights and observe both inside roosting associations and outside foraging encounters on herded cattle. Kinship estimates were calculated for all bats studied and used as predictors in the analysis of foraging encounters. Of the 50 bats tagged, 23 had previously been in captivity, and data on their food-sharing and social grooming interactions in captivity were also used as predictor variables for foraging behaviour.
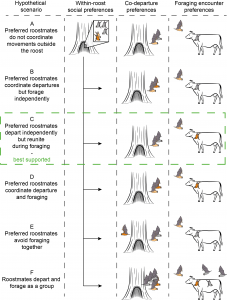
Figure 1 (from preprint). Hypothetical scenarios for the association between within-roost relationships and foraging behaviour. Preferred roostmates are shown as pairs of light brown and dark brown bats.
Key findings
The authors of the study tracked 50 individual vampire bats over several days and nights, aiming to reveal the relationship between social networks in the roost and foraging behaviour at night. In other words: which roostmates forage together, if any?
- The authors found that bats tend to leave the roost independently, but preferred roostmates often reunite while foraging, supporting Hypothesis C (Figure 1). Departures from the roost were based on tagged bats losing connection with the roost proximity sensor, and almost all departures were more than 5 seconds apart. The researchers similarly never saw large groups of bats leaving the nest together.
However, bats were observed foraging on cattle together, and these dyadic interactions were more likely amongst close kin and bonded roostmates (both in the wild or previously in captivity). The authors suggest that joint foraging might be more ‘cost-effective’ than food-sharing at the roost, illustrating another way in which long-term ties between bats are important for individual (and roost) fitness. - Social traits of individual bats are stable: bats who made many dyadic associations in the roost during the day (bats with high social network centrality) were also likely to maintain this centrality during the foraging period. The basis for this consistent variation between individuals is not explored here but could have implications for overall roost social structure.
- Time in captivity appears to affect bat foraging behaviour in the long term. Bats who had previously spent time in captivity departed the roost on average 1.6 hours earlier than wild bats, without an increase in time spent foraging. This effect was picked up thanks to the study design and is an intriguing aspect of bat behaviour to consider in future studies.
- The authors observed a previously unknown call type in vampire bats – an “n-shaped call”, distinct from the known “sweeping” and “buzz” calls. N-shaped calls were made by bats interacting near cattle, suggesting that they may be involved specifically in foraging encounters between preferred roostmates.
Why I liked this preprint
I was initially intrigued by this paper because of my interests in animal foraging and the evolution of social behaviour. Tracking individual bats, and indeed many free-ranging animals, has been historically challenging, and I thought the approach here was well-suited to answering the research questions. I really liked the clear explanation and figure demonstrating alternative hypotheses for foraging associations between roostmates. I was especially intrigued by the discovery of a new type of call and I am curious about the role it plays in roostmate communication and foraging. Overall, the paper provides interesting findings on the social structure and foraging behaviour of vampire bats, with implications for the evolution of cooperation in these animals.
Questions for the authors
- Do you think bats differentiate between non-preferred roostmates (i.e. the “grey” bats in Figure 1) and conspecifics from another roost? If so, are they differentiated by different calls? Is there an advantage to favouring non-preferred roostmates over non-roostmates?
- Do you know why some individuals are more highly connected than others? Are highly connected individuals different from other bats in physical traits, for example size?
- You state that dominance hierarchies among female vampire bats are not necessarily clear and linear. How is social dominance determined and manifested amongst vampire bats and did you investigate dominance in the bats you studied? Do you think that dominance is also a stable trait within individuals?
Further reading
Wilkinson GS, 1984. Reciprocal food sharing in the vampire bat. Nature 308:181.
doi: https://doi.org/10.1242/prelights.29227
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Responses to conflicting binocular stimuli in mouse primary visual cortex
Maitri Manjunath
Effects of transcranial photobiomodulation on peripheral biomarkers associated with oxidative stress and complex IV activity in the prefrontal cortex in rats subjected to chronic mild stress
Rickson Ribeiro, Marcus Oliveira
Psychedelics Align Brain Activity with Context
Loïk Holdrinet et al.
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |











 (No Ratings Yet)
(No Ratings Yet)