Aspergillus dsRNA virus drives fungal fitness and pathogenicity in the mammalian host
Posted on: 13 May 2024
Preprint posted on 17 February 2024
A mycovirus could be the Achilles Heel of Aspergillus fumigatus; curing it could cure us.
Selected by UofA IMB565, Jennifer Uhrlaub, Christina Arnoldy, Anh Evy NguyenCategories: microbiology
Background
Fungal pathogens represent a significant and underappreciated threat to global health. The diversity of pathogenic fungi is vast, with these organisms inhabiting diverse ecological niches and causing a wide array of diseases in humans. Aspergillus species alone put over 30 million people at risk of invasive aspergillosis annually, with more than 300,000 patients developing this infection each year (1).
Aspergillus fumigatus (A. fumigatus) is a saprotrophic fungus primarily found in soil. It is known for its high mortality rates when it infects humans, especially immunocompromised individuals. A. fumigatus produces gliotoxin and causes the respiratory disease known as aspergillosis, which results in asthma, lung tissue necrosis, and pneumonia (2).
With great diversity, both in terms of species and pathogenesis, diagnosis and treatment for fungal diseases are complicated matters. There are limited antifungal drugs available, and their efficacy varies depending on the specific fungus involved. The emergence of drug-resistant strains complicates the already limited treatment options, warranting research and the discovery of new strategies.
A change in perspective that may reveal new treatment strategies is viewing pathogens as hosts themselves. Fungal species that are pathogenic in humans are themselves hosts to mycoviruses. In this preprint, the authors explore how A. fumigatus polymycovirus-1 (AfuPmV-1M), a small dsRNA virus, enables A. fumigatus to resist oxidative stress, heat shock, and low pH for better survival and replication in all the environments of its life cycle from dirt to lung. Antiviral treatment to cure A. fumigatus of this mycovirus could attenuate the ability of the fungus to infect humans. As such, AfuPmV-1M could be the Achilles heel that could be targeted to take down this fungal pathogen.
Key findings
Mycovirus AfuPmV-1M infection provides growth advantages to A. fumigatus
The authors compared the replicative ability and virulence of A. fumigatus infected with AfuPmV-1M to that of virus-cured and re-infected. They found that infected and re-infected strains produced more conidia and that these conidia maintained higher viability under conditions of oxidative stress and heat, features of the soil environment where A. fumigatus is found. To begin to characterize how AfuPmV-1M alters its host fungus, the authors performed proteomics, revealing an upregulation of proteins that localize with stress granules (SG). Thus, the authors propose that by increasing the expression of SG components, AfuPmV-1M may enhance SG formation, better preparing the fungus to withstand the stresses of life both within its natural environment and within a host.
Mycovirus infection boosts A. fumigatus survival in phagocytes
Further experiments investigated the effect of AfuPmV-1M on A. fumigatus in murine and cellular models of infection. Co-infection of mice with each of the three strains (Virus-Infected, Re-infected and Virus-Cured), distinguished by cell wall labeling, yielded lung neutrophils containing phagocytosed fungi. This model of infection avoided potential confounding effects of intrinsic variation between mice. Colony forming unit analysis showed that virus-infected conidia survived better in murine- and human- derived lung neutrophils and in Acanthamoeba castellanii, phagocytes that A. fumigatus encounters in soil.
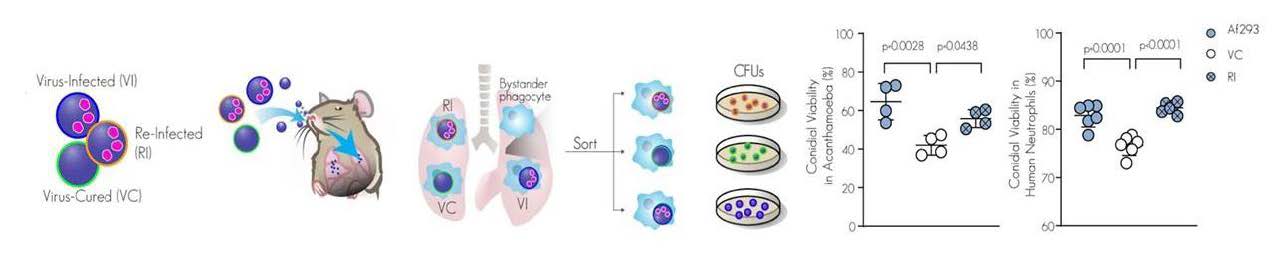
A. fumigatus virulence is enhanced by mycovirus AfuPmV-1M infection
Mice challenged with virus-infected A. fumigatus had higher fungal burden, inflammation, and mortality as compared to mice infected with virus-cured fungi. However, this difference in virulence was nullified in mice deficient in NADPH oxidase 2, an enzyme responsible for maintaining the oxidative environment of phagosomes. The authors also demonstrated that therapeutic treatment of A. fumigatus infected mice with the antiviral ribavirin significantly improved survival, supporting their idea that the virus within the fungus is a feasible target to reduce the lethality of aspergillosis.
What we like about this preprint
We especially liked the co-infection strategy of virus-infected, -cleared, and -reinfected A. fumigatus. The pairing of fluorescently labeled fungi and simple fluorescence-activated cell sorting of infected neutrophils cleverly demonstrated that polymycovirus infection allows the fungi to better survive phagocytosis. Taken together with other data presented, the authors clearly show that AfuPmV-1M confers resistance to external stress conditions and exacerbates fungal virulence in mammalian hosts. This ultimately demonstrates that what doesn’t kill you makes you stronger and more dangerous.
Future directions and questions for the authors
Application to human health and disease is an obvious next step. We suggest profiling clinical isolates of A. fumigatus for mycovirus infection correlated to disease severity. If severe disease is associated with mycovirus infection, it could be applied as a diagnostic predictor. To be a viable treatment option for aspergillosis, it remains to be formally demonstrated that Ribavirin reduces fungal burden without pre-treatment of the fungus itself. We also wonder about the prevalence of mycovirus infection in regions with harsh environmental conditions. Is climate change selecting for pathogenic fungi?
References
1. Fisher MC, Gurr SJ, Cuomo CA, Blehert DS, Jin H, Stukenbrock EH, et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. MBio [Internet]. 2020 May 5;11(3). Available from: http://dx.doi.org/10.1128/mBio.00449-20
2. Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clinical microbiology reviews. 2009 Jul;22(3):447-65.
doi: Pending
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)