Killing of Gram-negative and Gram-positive bacteria by a bifunctional cell wall-targeting T6SS effector
Posted on: 21 April 2021
Preprint posted on 4 March 2021
Article now published in Proceedings of the National Academy of Sciences at http://dx.doi.org/10.1073/pnas.2106555118
Le et al. show how Acinetobacter baumannii can use a T6SS effector to kill both Gram-negative and Gram-positive competitors
Selected by NYUPeerReviewCategories: microbiology
Background
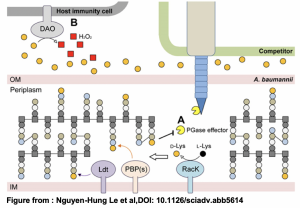
Numerous bacterial species use type VI secretion systems (T6SS) to antagonize neighboring Gram-negative bacteria. T6SSs are multi-protein macromolecular complexes that secrete toxins or other proteins to kill bacteria. This activity allows bacteria to compete for resources in complex bacterial communities in the environment and in an animal host. Acinetobacter (A.) baumannii is a member of the ESKAPE pathogens, which are highly virulent and antibiotic resistant organisms that affect immunocompromised hosts. In their preprint, Nguyen-Hung Le at al. show that the Gram-negative bacterium A. baumannii modulates its extracellular milieu in order to potentiate the activity of a T6SS effector, Tse4. Furthermore, the authors found Tse4 can kill both Gram-negative and Gram-positive bacteria. Understanding how A. baumannii competes with other species may allow to better control outbreaks of this deadly species.
Key findings
- A. baumannii was known to secrete high levels of the non-canonical amino acid D-lysine.
- Secretion of D-Lysine in the extracellular milieu optimized the enzymatic activity of Tse4, which requires an alkaline pH for optimal activity.
- The authors revealed that Tse4 has both endopeptidase and lytic transglycosylase activities.
- Tse4 orthologues are present in various bacterial species, suggesting the importance of this family of T6SS effectors in bacterial warfare.
This work showed many “firsts”. First, the secretion of a non-canonical amino acid plays a key role in the activity of a secreted protein effector. Additionally, Tse4 was shown to have two types of cell wall (peptidoglycan) hydrolase activities. Perhaps most strikingly, the authors showed that Tse4 could affect Gram-positive bacteria.
Why we chose this preprint
Acinetobacter baumannii is a high priority pathogen due to its appearance in hospital settings where vulnerable populations can be affected. T6SS were discovered in the past 15 years therefore the mechanics of how they function are relatively unknown. Furthermore, previously it was known that non-canonical amino acids can be abundantly secreted from bacteria but their roles in biology were unknown. This work demonstrates a rare example of connecting amino acid secretion to the activity of a toxic secreted enzyme. Finally, the authors show that this enzyme has activity against Gram-positive as well as Gram-negative bacteria.
Questions for the authors
- Could the authors share their thoughts on how they imagine this sophisticated way of killing to work in a more complex and realistic bacterial system where there are more predators and preys, i.e., is racK activity regulated?
- Do the other “prey” bacteria utilized in this preprint secrete D-Lys? How does this affect the competition between A. baumanii and other “prey” bacteria?
- Are the activities (lytic transglycosylase and endopeptidase) of Tse4 due to the same active site? Is/are the active site(s) known?
References
- Le, N.-H., Peters, K., Espaillat, A., Sheldon, J. R., Gray, J., Di Venanzio, G., Lopez, J., Djahanschiri, B., Mueller, E. A., Hennon, S. W., Levin, P. A., Ebersberger, I., Skaar, E. P., Cava, F., Vollmer, W., & Feldman, M. F. (2020). Peptidoglycan editing provides immunity to Acinetobacter baumannii during bacterial warfare. Science Advances, 6(30), eabb5614.
- Russell, A. B., Peterson, S. B., & Mougous, J. D. (2014). Type VI secretion system effectors: poisons with a purpose. Nature Reviews. Microbiology, 12(2), 137–148.
doi: https://doi.org/10.1242/prelights.28501
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)