Lytic bacteriophages interact with respiratory epithelial cells and induce the secretion of antiviral and proinflammatory cytokines
Posted on: 20 May 2024
Preprint posted on 6 February 2024
Phage therapy FTW!💥 But be warned, the vibes that these efficient bacterial killers have with human cells can vary!🧫 Get the scoop on these micro-warriors!💊 #PhageRevolution
Selected by UofA IMB565, Riley Hellinger, Yash SharmaCategories: microbiology
Background
The prevalence of antibiotic resistance has continued to rise globally, leading to an increased demand for new therapeutic options (1). However, there has been a lack of new antibiotic compounds being developed (2), leaving a gap for alternative therapeutic approaches to emerge. In response, bacteriophages (phages), viruses that infect bacteria with high specificity (3), have been used for decades in Eastern European countries to combat infections (4). In more recent years, Western countries, such as the United States, have slowly began using phages in a therapeutic context, though this has thus far only been via clinical trials and Emergency Use Authorizations (5). In these cases, phage therapy has elicited minimal side effects and has generally been considered to be safe for use (6).
One opportunistic pathogen that phage therapy has shown promise with is Pseudomonas aeruginosa. There is a dire need for new therapies against this pathogen which greatly impacts those with compromised immune systems, such as patients with cystic fibrosis (7). P. aeruginosa antibiotic resistance has become increasingly prevalent, with some strains developing resistance to nearly all antibiotics (8). In patients with cystic fibrosis, infection with this bacterium has been correlated with decreased lung function, eventually leading to respiratory failure and death (9).
Phages used in therapy are generally chosen for their specificity to a specific bacterial strain and for their use of a lytic lifecycle, which allows them to efficiently kill bacteria that become infected (3). While phages do not infect and cannot replicate within a mammalian cell (3), previous studies have shown that they can interact and be taken up by eukaryotic cells (10-12). This had led to some concerns about phage therapy since phages are usually administered in high doses to kill off the pathogenic bacteria (3, 13, 14). In order to design phage therapies that will effectively clear a bacterial infection without causing unexpected or adverse responses in the patient, more work needs to be done to understand how human tissues and the immune system sense and respond to phages (15). This is especially important as phages are incredibly diverse in features such as size and morphology, indicating a potential for diverse responses.
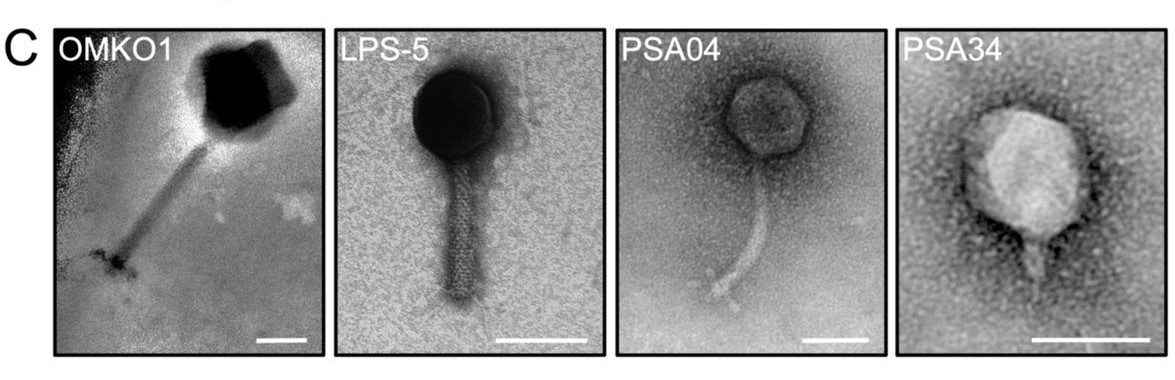
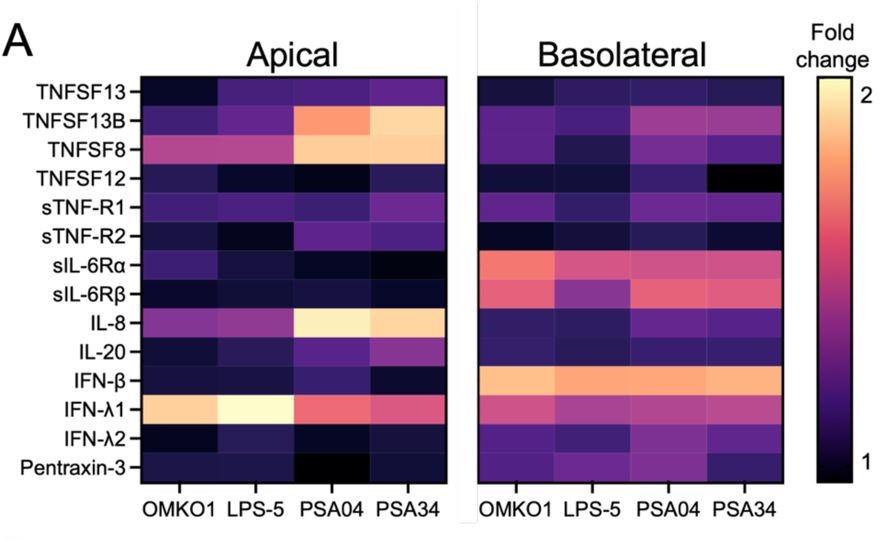
In this preprint, the authors have studied a panel of four phages that infect various P. aeruginosa but have diversity in their size and morphologies (Figure 1A). They show that these phages directly interact with human bronchial epithelial cells from a patient with cystic fibrosis. Additionally, the airway epithelial cells sense and mount antiviral responses in a phage-specific manner (Figure 1B). Overall, they conclude that testing phages for their interaction with mammalian cells and the cellular response induced by this interaction should be included in the phage therapy development pipeline.
Key Findings
Individual bacteriophages interact with the respiratory epithelium in unique ways.
After morphological and genetic characterization of four clinically relevant lytic phages that were specific for different strains of P. aeruginosa (OMKO1, LPS-5, PSA04, and PSA34), the authors sought to determine how these phages interact with the respiratory epithelium. By using plaque assays and confocal microscopy, they found that an acidic pH, which is characteristic of airways in patients with cystic fibrosis and other inflammatory disorders, promotes phage-epithelium association. They observed that under these conditions, myoviridae phages (OMKO1 and LPS-5) tended to significantly increase their association with the apical surface. In contrast, the myoviridae phages did not form aggregates on the surface while PSA04 and PSA34 did. Additionally, the phages were able to penetrate into the epithelium, without disrupting epithelial integrity or causing cytotoxicity, but their distribution appeared to be variable depending on phage size and morphology. OMKO1, the largest of the phages tested, was not detected in the basolateral compartment while all the others were detected. From these data, the authors concluded that the interaction dynamics between the human epithelium and therapeutical phages will likely be phage-specific.
Airway epithelial cells recognize phages as pathogen associated molecular patterns (PAMPs).
Next, the authors sought to determine if airway epithelial cells were able to detect and initiate a response to the phages using a HEK293 cell toll-like receptor reporter system, RNA sequencing and by measuring levels of secreted cytokines. By doing so, they found that all the phages were detected by the airway epithelial cells via toll-like receptors. The RNA sequencing results revealed that genes of the tumor necrosis factor superfamily were significantly upregulated, suggesting that the phages had indeed been detected as pathogens.
Cytokines are secreted by airway epithelial cells in response to phage exposure.
Finally, the researchers hypothesized that cytokines would be secreted by airway epithelial cells in response to phage exposure and that these cytokines would be phage-specific. They observed that cytokines were mainly secreted apically and that they were specific to the phages. The myovirus-exposed cells secreted IFN-λ1 while those exposed to PSA04 and PS34 secreted TNFSF13B, TNFSF8, and IL-8. Interestingly, of the cytokines analyzed, all phages induced basolateral secretion of IFN-β. Together, these data indicate that an antiviral response was initiated by airway epithelial cells exposed to phages, though the response was phage-specific.
Why we chose this preprint
We chose this preprint because of the growing importance of multidrug resistant infections and the lack of new antibiotic development. This study is particularly interesting as it addresses an important, yet understudied, question in the field of bacteriophage therapy: how do human cells and tissues sense and interact with phages? The authors of this preprint were able to conclude that the interactions between phages and human cells/tissues is another factor that should be included in the criteria for phage therapy selection. This inclusion may allow for highly effective clearance of the bacterial pathogen with minimal adverse effects on the patient.
Future Directions
Further research could delve into elucidating the specific molecular mechanisms underlying the interactions between lytic bacteriophages and respiratory epithelial cells. Understanding the signaling pathways and immune responses triggered by phage exposure could offer valuable insights into the potential therapeutic applications of phage therapy in respiratory infections. Additionally, exploring the long-term effects of phage-induced cytokine secretion on respiratory health and immune function could help in perfecting treatment strategies and minimizing potential adverse outcomes. Furthermore, investigating the potential synergistic effects of combining phage therapy with other antimicrobial agents or immunomodulators could open new avenues for enhancing the efficacy of treatment regimens against multidrug-resistant infections.
Questions for the Authors
Q1- What criteria were used to initially select the four phages (OMKO1, LPS-5, PSA04, and PSA34) from the larger panel for further characterization?
Q2- Do you plan to follow this up with a larger panel of phages, such as those remaining from your initial panel of P. aeruginosa phages and/or with phages that have hosts of completely different genera to see if you can identify any sort of trends?
Q3-How were the transcriptomic analyses (Figures 6 and 7) performed, and what were the key pathways or genes identified as differentially expressed upon phage treatment?
Q4-How do the findings from this study inform the selection criteria for choosing optimal phages for therapeutic applications, beyond just considering their bacterial host range?
Q5-If phages enter the bloodstream, they could potentially be recognized as foreign particles by the systemic immune system, leading to an immune response against the phages themselves. This could result in anti-phage antibodies or other immune mediators, which may neutralize the phages and reduce their therapeutic efficacy or not?
Q6- Since responses to Pf phage had previously been tested in keratinocytes, why wasn’t it used in this study as a control to determine if the keratinocyte response would be similar to the response of AECs grown at an ALI?
doi: Pending
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the microbiology category:
Cryo-EM reveals multiple mechanisms of ribosome inhibition by doxycycline
Leonie Brüne
Microbial Feast or Famine: dietary carbohydrate composition and gut microbiota metabolic function
Jasmine Talevi
Citrobacter rodentium infection activates colonic lamina propria group 2 innate lymphoid cells
André Luiz Amorim Costa, Marcus Oliveira
preLists in the microbiology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
BioMalPar XVI: Biology and Pathology of the Malaria Parasite
[under construction] Preprints presented at the (fully virtual) EMBL BioMalPar XVI, 17-18 May 2020 #emblmalaria
| List by | Dey Lab, Samantha Seah |
1
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Antimicrobials: Discovery, clinical use, and development of resistance
Preprints that describe the discovery of new antimicrobials and any improvements made regarding their clinical use. Includes preprints that detail the factors affecting antimicrobial selection and the development of antimicrobial resistance.
| List by | Zhang-He Goh |











 (No Ratings Yet)
(No Ratings Yet)