Axon-like protrusions promote small cell lung cancer migration and metastasis
Posted on: 1 October 2019 , updated on: 2 October 2019
Preprint posted on 6 August 2019
Article now published in eLife at http://dx.doi.org/10.7554/eLife.50616
Neuron + cancer cell = deadly combination! Yang et al. find that cancers that exhibit a neuronal phenotype metastasize more.
Selected by Pavithran RavindranCategories: cancer biology
Background
Metastasis is the deadliest manifestation of cancer in which cancer cells are able to disseminate from the primary tumor and take up residence in a novel location within the body1–3. Such a process involves the intricate interplay of a number of molecular processes to allow the cancer cell to survive such a perilous journey4. Understanding this process is crucial to the development of molecular targets for cancer therapy. One poorly understood feature of, specifically, lung cancer is the expression of neuron-specific genes that seem to be upregulated in cancer cells that have metastasized. Does the manifestation of a neuronal-like phenotype aide cancer cells to metastasize? The authors in this preprint set out to investigate exactly this question.
Key Findings
To understand whether or not the expression of neuronal genes plays a role in metastasis, the authors decided to first determine whether or not lung cancer cells exhibit neuron-like phenotypes in culture and in vivo. When growing the cancer cells in a monolayer – by plating 3 lung cancer cell lines on Matrigel – and grafting them subcutaneously, the authors found that a subset of the cancer cells exhibited neuron-like protrusions (Figure 1). The authors then did a retrospective study and found a list of 70 candidate genes thought to be important in axon guidance, neuron growth and migration, and found that 69 of the 70 genes were present in small-cell lung cancer cells. The authors then stained these cells for axon/neuron specific genes and found that the protrusions exhibited many of these axon-specific genes. Finally, they confirmed that cells that had these protrusions were able to migrate faster than cells without them, very similar to the migration patterns of neuroblasts. Overall, these data suggest that cancer cells exhibit protrusions and these protrusions are very similar to axons growing off of neurons.
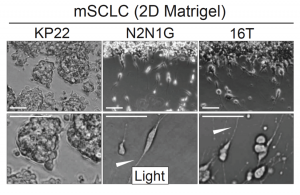
How are these protrusions affecting the metastatic potential of the cancer cells? The authors took the list of 70 genes and cut it down to a list of 13 genes that played non-overlapping roles in axon formation, guidance and neuronal migration. Upon analysis of the Cancer Dependency Map project, knockdown of these particular genes did not seem to have an effect on the growth of cancer cells in culture. However, when the authors used shRNAs to knock down these genes in N2N1G cells (mouse small-cell lung cancer cells that were derived from a lymph node metastasis), they found that these cells had fewer protrusions and migrated slower compared to wild-type cells. This further bolsters the idea that these protrusions are truly axon-like and the cells are behaving similarly to neuroblasts. Finally, the authors found that knockdown of two different genes that are important for the development of axons (Gap43 and Fez1) did not affect the growth of a primary tumor but significantly affected the metastatic potential of the cells, as mice implanted with tumors with shRNAs to these genes had much fewer metastases. Overall, this work suggests that protrusions that seem to grow in small-cell lung cancers are axon-like and play an important role in the metastatic potential of the cells.
Why I chose this preprint
Metastasis is a very diverse problem in which multiple programs are initiated in cancer cells with the end goal of colonizing a new organ. How this process takes place has been the focus of many papers in the past decade. What this paper does quite elegantly is find a phenotypic difference of cancer cells compared to normal cells and characterize how such a phenotype and its genetic drivers may actually contribute to this deadly process. This work is even more interesting in the context of recent papers published this month in which labs have shown that glioblastoma cells may acquire neuron like features and interface with normal neurons to grow5–7.
Questions for the authors
1. You hypothesize that the neuronal-like axons growing from tumor cells may help in the initial steps of metastasis. After engrafting a primary tumor, did you extract the blood from the mice and see if there are less cancer cells there after shRNA knockdowns?
2. Since these cells seem to be acting like neuroblasts, did you notice any correlation between the phenotype and brain metastases?
References
- Chen, Q. et al. Carcinoma – astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature (2016). doi:10.1038/nature18268
- Boire, A. et al. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis Article Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell 1101–1113 (2017). doi:10.1016/j.cell.2017.02.025
- Zhang, X. H. F. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 (2013).
- Lawson, D. A., Kessenbrock, K., Davis, R. T., Pervolarakis, N. & Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 20, 1349–1360 (2018).
- Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019).
- Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
- Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532-538 (2019).
doi: https://doi.org/10.1242/prelights.14285
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the cancer biology category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Taxane-Induced Conformational Changes in the Microtubule Lattice Activate GEF-H1-Dependent RhoA Signaling
Vibha SINGH
ROCK2 inhibition has a dual role in reducing ECM remodelling and cell growth, while impairing migration and invasion
Sharvari Pitke
preLists in the cancer biology category:
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
February in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry and cell metabolism 2) cell organelles and organisation 3) cell signalling, migration and mechanosensing
| List by | Barbora Knotkova et al. |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
CellBio 2022 – An ASCB/EMBO Meeting
This preLists features preprints that were discussed and presented during the CellBio 2022 meeting in Washington, DC in December 2022.
| List by | Nadja Hümpfer et al. |
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
Single Cell Biology 2020
A list of preprints mentioned at the Wellcome Genome Campus Single Cell Biology 2020 meeting.
| List by | Alex Eve |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Anticancer agents: Discovery and clinical use
Preprints that describe the discovery of anticancer agents and their clinical use. Includes both small molecules and macromolecules like biologics.
| List by | Zhang-He Goh |
Biophysical Society Annual Meeting 2019
Few of the preprints that were discussed in the recent BPS annual meeting at Baltimore, USA
| List by | Joseph Jose Thottacherry |











 (No Ratings Yet)
(No Ratings Yet)