A zebrafish circuit for behavioral credit assignment
Posted on: 3 March 2025 , updated on: 4 March 2025
Preprint posted on 13 February 2025
Neural blueprint for credit assignment in zebrafish: linking specific actions to outcomes via a dorsal habenula–interpeduncular circuit.
Selected by Muhammed Sinan MalikCategories: animal behavior and cognition, neuroscience
Background:
Learning to associate an action with its outcome, “credit assignment”, is a fundamental challenge for all brains. In both natural and artificial systems, the problem is how to pinpoint which neuronal circuit elements deserve credit (or blame) for a successful or failed action. Classic studies in dopamine signalling and prediction error paved the way for understanding reinforcement learning 1. In mammals, the lateral orbitofrontal cortex (lOFC) and hippocampus (HC) are known to form abstract representations that help assign credit to specific choices, even when the feedback is delayed 2,3.
Recent theoretical and experimental advances have begun to unravel the cellular and circuit-level mechanisms underlying this process. In zebrafish, a vertebrate model that enables optical and genetic access to neural circuits, researchers have identified a key pathway for credit assignment: the dorsal habenula (dHb) to interpeduncular nucleus (IPN) circuit (see Fig.1) 4,5.
This pathway is particularly intriguing because it is lateralized. The right dHb, enriched for neurons expressing the lratd2a gene, receives bilateral olfactory input from mitral cells and selectively projects to a defined region of the IPN 6,7. Such asymmetry is not unique to zebrafish; lateralized processing of sensory cues is observed in bees8, rodents9 and humans 10. Thus, deciphering how the right dHb integrates sensory (e.g. olfactory, visual etc) cues with motor actions may illuminate a conserved mechanism by which vertebrate brains assign credit.
In the present preprint 4, the authors combine innovative operant behavioral assays with calcium imaging, chemogenetic manipulations, and detailed circuit reconstructions (Fig. 1) to show that adaptive responses in zebrafish require a precise temporal coupling between an action (a turn) and its sensory feedback (temperature change). Notably, they demonstrate that the dHb–IPN circuit uses GABAB receptor–mediated presynaptic modulation to “multiply” sensory and choice signals effectively marking the synapses that contributed to a successful action 11,12.
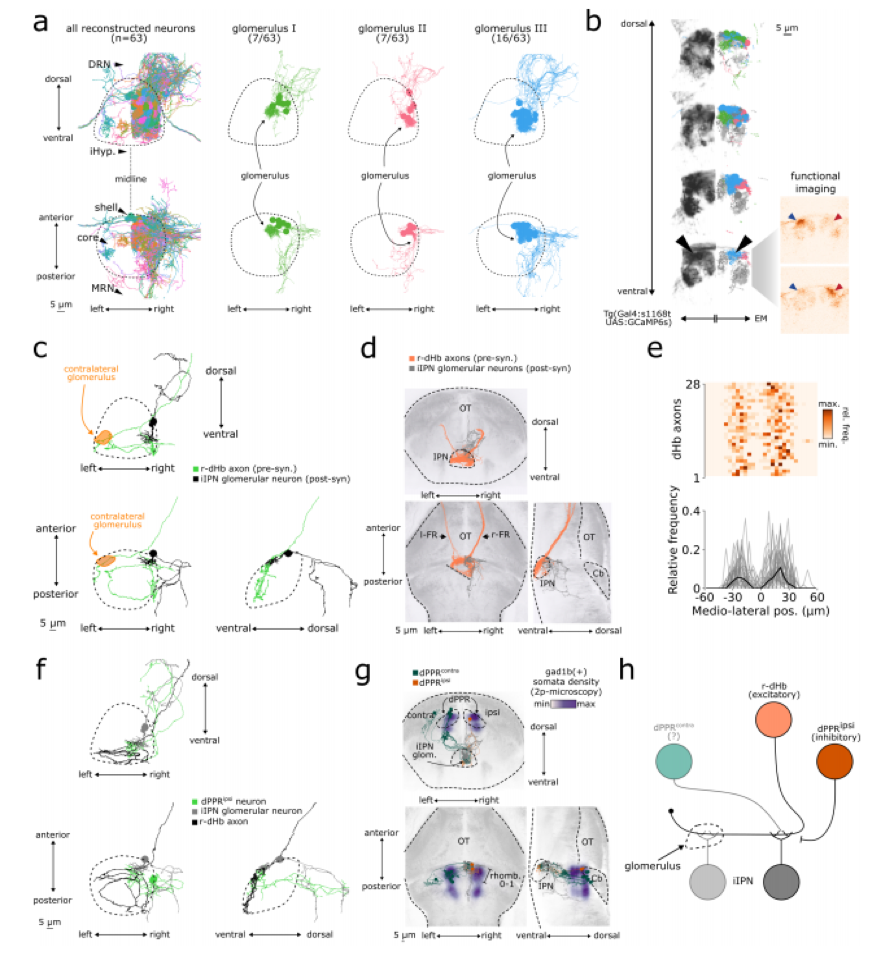
Fig. 1 – Anatomical reconstructions and circuit organization (a–h) illustrating the connectivity of dorsal habenula (dHb) axons with glomerular inhibitory interpeduncular nucleus (iIPN) neurons. Fig. 3 of the preprint highlighted here, made available under a CC-BY-NC-ND 4.0 International license.
Key Findings:
Temporal Coupling is Essential:
Using an operant thermoregulatory assay, the authors showed that zebrafish must execute a turn and then receive a temperature boost (tempUP) within a narrow time window to reduce exposure to cold. Delays disrupted this credit assignment process, indicating that precise timing is crucial for learning which actions lead to positive outcomes.
Circuit Specificity – The dHb–IPN Pathway:
Through chemogenetic ablations, the authors demonstrated that the dorsal habenula—particularly its right subregion rich in lratd2a-expressing neurons—is essential for adaptive behavior. Although these neurons are not required for innate temperature responses, they are indispensable for experience-dependent behavioral adjustments.
Multiplicative Integration via GABAB Modulation:
Functional imaging revealed that neurons in the intermediate IPN act as coincidence detectors: they produced a robust, multiplicative-like response only when a specific turn was closely followed by a temperature increase. Pharmacological blockade of GABAB receptors abolished these responses, underscoring their role in presynaptic modulation of dHb axon terminals.
Lateralization and Generalization:
The asymmetry observed in the zebrafish dHb mirrors lateralized processing seen in other species. This not only reinforces the importance of the right dHb in linking actions to outcomes, but also suggests that similar circuit-level solutions may be used across vertebrates for credit assignment in both olfactory and other sensory modalities.
Why I highlight this preprint?
This paper elegantly dissects the circuit-level mechanisms underlying credit assignment, an essential computation for adaptive behavior, by combining innovative operant assays with high-resolution imaging and chemogenetic manipulations in zebrafish. I really appreciate the way in which the study convincingly describes a precise temporal integration of motor actions with sensory feedback via the dorsal habenula–interpeduncular pathway, highlighting the role of presynaptic GABAB receptor modulation. This multidisciplinary approach not only deepens our understanding of neural computations but also offers a compelling framework for exploring similar mechanisms across vertebrates, aligning perfectly with my research interests in unraveling the neural basis of adaptive behavior.
Questions for the authors:
- Can the same dHb–IPN circuit mechanism support credit assignment for other sensory feedback modalities (e.g., visual or auditory cues), or is it specialized for thermosensory inputs?
- Given the critical dependence on precise timing between an action and its feedback, how sensitive is the circuit to small variations in the action–outcome interval, and what cellular mechanisms might ensure that this narrow temporal window is maintained?
- Since dHb ablation impairs adaptive responses but spares innate temperature reactions, how do you envision the dHb differentially integrating experience-dependent signals compared to hardwired sensorimotor circuits?
References:
- Schultz, W., Dayan, P., & Montague, P. R. (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593-1599.
- Boorman, E. D., Behrens, T. E., & Rushworth, M. F. (2011). Counterfactual choice and learning in a neural network centered on human lateral frontopolar cortex. PLoS biology, 9(6), e1001093.
- Jocham, G., Klein, T. A., & Ullsperger, M. (2011). Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. Journal of Neuroscience, 31(5), 1606-1613.
- Paoli, E., Palieri, V., Shenoy, A., & Portugues, R. (2025). A zebrafish circuit for behavioral credit assignment. bioRxiv, 2025-02.
- Cherng, B. W., Islam, T., Torigoe, M., Tsuboi, T., & Okamoto, H. (2020). The dorsal lateral habenula-interpeduncular nucleus pathway is essential for left-right-dependent decision making in zebrafish. Cell reports, 32(11).
- Miyasaka, N., Arganda-Carreras, I., Wakisaka, N., Masuda, M., Sümbül, U., Seung, H. S., & Yoshihara, Y. (2014). Olfactory projectome in the zebrafish forebrain revealed by genetic single-neuron labelling. Nature communications, 5(1), 3639.
- Decarvalho, T. N., Akitake, C. M., Thisse, C., Thisse, B., & Halpern, M. E. (2013). Aversive cues fail to activate fos expression in the asymmetric olfactory-habenula pathway of zebrafish. Frontiers in neural circuits, 7, 98.
- Rogers, L. J., & Vallortigara, G. (2008). From antenna to antenna: lateral shift of olfactory memory recall by honeybees. PLoS One, 3(6), e2340.
- Sakaguchi, Y., & Sakurai, Y. (2017). Left–right functional asymmetry of ventral hippocampus depends on aversiveness of situations. Behavioural Brain Research, 325, 25-33.
- Avram, J., Balteş, F. R., Miclea, M., & Miu, A. C. (2010). Frontal EEG activation asymmetry reflects cognitive biases in anxiety: evidence from an emotional face Stroop task. Applied psychophysiology and biofeedback, 35, 285-292.
- Amo, R., Fredes, F., Kinoshita, M., Aoki, R., Aizawa, H., Agetsuma, M., … & Okamoto, H. (2014). The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron, 84(5), 1034-1048.
- Noonan, M. P., Chau, B. K., Rushworth, M. F., & Fellows, L. K. (2017). Contrasting effects of medial and lateral orbitofrontal cortex lesions on credit assignment and decision-making in humans. Journal of Neuroscience, 37(29), 7023-7035.
doi: https://doi.org/10.1242/prelights.39753
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)