DNA methylation enables recurrent endogenization of giant viruses in an animal relative
Posted on: 27 February 2024 , updated on: 28 February 2024
Preprint posted on 8 January 2024
Article now published in Science Advances at http://dx.doi.org/10.1126/sciadv.ado6406
Categories: evolutionary biology
Background
Methyl groups can be added to DNA bases via enzymes called DNA methyltransferases (DNMTs). These modifications can change how a gene is expressed (i.e., enhance or repress). DNMTs are found in many eukaryotes and are generally well conserved throughout eukaryotic evolution. 5-methyl cytosine (5mC) is generated when DNMTs add a methyl group from S-adenosyl methionine (SAM) to cytosine on the 5th carbon of its ring (1). Typically, 5mC in eukaryotes relates to gene silencing, and these modifications are often associated with DNA sequences originating from viruses and transposable elements (TE). Silencing these exogenous DNA elements can prevent them from becoming toxic to their host. During a viral infection, DNA from the virus can incorporate into the host’s DNA cell and may be left behind long after the infection is cleared. This could, for example, happen after giant virus infections. These viruses have large genomes and invade many kinds of eukaryotic cells. Evidence suggests that some genes important for eukaryotic evolution originate from these large viruses whose DNA was incorporated into the genome of the host cell (2). However, how eukaryotes incorporate and keep viral DNA sequences in check to stop them causing problems is unclear. In this study, the authors show that in a close relative of animals, a protist called Amoebidium appalachense, 5mC silences giant virus DNA present in their genome, preventing the viral DNA from becoming toxic to the cell.
Key Findings:
1) In A. appalachense, hypermethylated regions correlate with the locations of viral DNA insertions
Amoebidium appalachense is a protist relative of animals isolated from freshwater arthropods. Using short and long-read sequencing in addition to micro-C, (a technique used to capture information about chromosome-chromosome interactions) the authors assembled the A. appalachense genome, identifying 18 DNMTs in total, some of which can produce 5mC. After mapping DNA methylation across the genome, they discovered a pattern between areas with high methylation and the locations of transposable elements (TEs). In addition, high levels of methylation coincided with repeated sequences originating from 1) Giant viruses, 2) Adintoviruses (similar to virophages; may parasitize giant viruses), 3) Plavanka associated transposons. They estimated that 3.1% of the total genome of A. appalachense is composed of these three repetitive DNA species with ~ 14 % of the proteome derived from these viral-origin genes.
2) 5mC keeps viral DNA in check in A. appalachense
To understand what these endogenized viral DNA sequences might offer A. appalachense, the authors rebuilt the gene repertoire of these giant viruses and examined which of the viral genes were incorporated into the host genome, finding many genes enriched for viral integration, replication, regulation, and transporters; all of which likely relate to ‘viral takeover’ of a host cell during infection. Additionally, 10 of the 18 DNMTs were of viral origin suggesting that they may could have been used by the virus, though for what purpose is currently unknown but perhaps for gene expression control – giant viruses have the capacity to methylate their own DNA (3) – or modulating interactions with the host immune system.
Additionally, the authors evidence for the presence of histone demethylases; enzymes that remove DNA methyl groups. These histone demethylases were enriched for a domain called Jumonji C (JmjC). These JmjC containing proteins are probably distant paralogs of histone lysine demethylases (KDM), subfamily 4 (KDM4) A. appalchense already has an ortholog of KDM4 in addition to these JmjC containing proteins. Interestingly, the underlying genes have a similar structure to a eukaryotic gene – containing multiple exons – which is rare in giant viruses. Some of these JmjC encoding genes are transcribed by A. appalachense suggesting they perform functions for this protist. The authors suggest two potential origins of these JmjC containing genes: 1) They are A. appalachense specific paralogs of these KDM4 genes that were acquired by the infecting giant virus, or 2) These KDM4-like genes came from an infected host, were incorporated into the giant virus, remodelled for use and then transferred back to A. appalachense during infection. The authors suggest that these KDM4-like enzymes could have helped the virus to avoid silencing by the host or aid viral incorporation into the host genome.
3) 5mC silences these viral DNA sequences
To investigate what role(s) 5mC plays in A. appalachense, the authors chemically depleted DNA methylation in these protozoans (from ~ 40 % to ~ 15 %). In methylation depleted samples, almost all TEs and genes associated with hypermethylated promoters were up regulated. Additionally, they detected transcripts originating from viral genes in the A. appalachense genome however, as no viral particles were found, there is not enough information left in the genome to create a fully functional new virus. Thus, 5mC minimises the risks of these viral sequences encompassed within the A. appalachense genome. Finally, the authors note that these incorporation events appear stochastic, probably from failed infections.
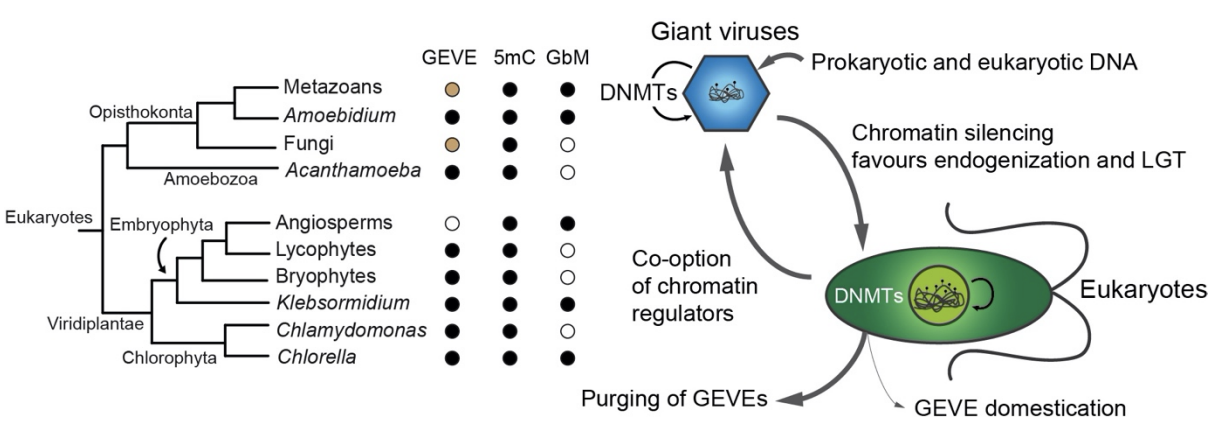
Figure adapted from Sarre et al. Figure summarises the relationship between 5mC in eukaryotic organisms and the endogenisation of giant viruses.
What I liked about this preprint:
I find it curious that eukaryotic genomes contain genes of viral origin and that these genes may be central to our evolution. Not only is A. appalachense an interesting organism in its own right but studying these unorthodox eukaryotes further often gives us very key insights into how complex biology evolves.
Questions for the authors:
1) Is 5mC the sole method used by A. appalachense to facilitate viral gene silencing?
2) How is 5mC reversed in A. appalachense? Do they have TETs like humans that trigger removal of the base via Base Excision Repair (BER)? Does the hypermethylation of these regions in this organism’s genome lead to increased DNA instability?
3) Could you speculate as to the potential relevance of gene body methylation in this organism given the correlations in animals between gene body methylation and transcription?
4) You demonstrate that A. appalachense possesses a large amount of viral DNA. As full viral particles cannot form based on the remaining sequences, could you speculate as to why this protist retained such a large quantity of potentially harmful DNA? What are the risks of expressing a viral replication factor for example? Do they outcompete the hosts?
References:
- Turpin, M & Salbert, G. 5-methylcytosine turnover: Mechanisms and therapeutic implications in cancer. Fron. Mol. Biosci. 2022
- Cheng, S., Wong, G.K-S, & Melkonian, M. Giant DNA viruses make big strides in eukaryotic evolution. Cell Host & Microbe. 2021.
- Jeudy, S., Rigou, S., Alempic, J-M., Claverie, J-m., Abergel, C, & Legendrem M. The DNA methylation landscape of giant viruses. Nat. Com. 2020
doi: https://doi.org/10.1242/prelights.36588
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the evolutionary biology category:
A drought stress-induced MYB transcription factor regulates pavement cell shape in leaves of European aspen (Populus tremula)
Jeny Jose
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
A high-coverage genome from a 200,000-year-old Denisovan
AND
A global map for introgressed structural variation and selection in humans
Siddharth Singh
preLists in the evolutionary biology category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
Planar Cell Polarity – PCP
This preList contains preprints about the latest findings on Planar Cell Polarity (PCP) in various model organisms at the molecular, cellular and tissue levels.
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
COVID-19 / SARS-CoV-2 preprints
List of important preprints dealing with the ongoing coronavirus outbreak. See http://covidpreprints.com for additional resources and timeline, and https://connect.biorxiv.org/relate/content/181 for full list of bioRxiv and medRxiv preprints on this topic
| List by | Dey Lab, Zhang-He Goh |
1
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Pattern formation during development
The aim of this preList is to integrate results about the mechanisms that govern patterning during development, from genes implicated in the processes to theoritical models of pattern formation in nature.
| List by | Alexa Sadier |











 (No Ratings Yet)
(No Ratings Yet)