DNA methylation rates scale with maximum lifespan across mammals
Posted on: 13 June 2023 , updated on: 14 June 2023
Preprint posted on 15 May 2023
Article now published in Nature Aging at http://dx.doi.org/10.1038/s43587-023-00535-6
Tik-Tok of the Methylation clock: does DNA methylation impact on our lifespan?
Selected by Jennifer Ann BlackCategories: genetics
Background:
Eukaryotes are capable of regulating gene expression in many ways, including via epigenetic changes. Epigenetic changes are broadly defined as chemical modifications that may alter gene expression but do not change the underlying DNA sequence (1). DNA methylation is a type of epigenetic change that involves specialised enzymes called methyltransferases which add methyl groups (-CH3) to specific regions of DNA. A common example is the methylation of the 5th carbon of the DNA base cytosine (C), generating 5-methylcytosine (or 5mC). When this cytosine is directly followed by a guanine (G), we refer to this combination of DNA bases as a CpG site (or 5’-C-phosphate-G-3’). By modifying the cytosine base in this manner, eukaryotes can alter the gene expression of the methylated DNA sequence. Curiously, the patterns of DNA methylation at these CpG sites that emerged correlate with ageing, suggesting we each possess an internal methylation-associated ‘molecular clock’. Additionally, aberrant DNA methylation can occurs in cancers (2). Thus, by diving deeper into DNA methylation, we may gain important insights not only into how organisms age and develop diseases, but potentially how we could encourage healthy ageing.
In this preprint article, Crofts et al. focus specifically on the role of DNA methylation in ageing, investigating whether there is a scaling relationship between the rate of DNA methylation and maximum lifespan across a range of different eukaryotic species. A scaling law mathematically describes an association between two physical quantities over several orders of magnitude and can reflect shared evolutionary constraints across species.
Key Findings:
1) Increased DNA methylation rates in conserved sites is associated with lower maximum lifespan
First, the authors examine DNA methylation rates within the blood of 7 different species of mammal. They selected species that reflect a range of maximum lifespan lengths, with the house mouse (less than 5 years) as the organism with the shortest lifespan to humans with the longest (over 100 years). To do this, they focus on a carefully selected group of conserved CpG sites whose methylation has been shown to correlate with ageing (i.e., age-related CpGs). They show that:
- The shorter the maximum lifespan of an organism, the higher the rate of DNA methylation in blood or in skin samples
- This relationship is not influenced by the size of the animal (i.e it’s body mass), which is unusual as many previously described scaling relationships with lifespan have been driven by mass.
What does this mean? This means that the maximum lifespan and the rate of DNA methylation scale proportionally i.e, if animal X lives 100 x longer than animal Y, the rate of DNA methylation for animal X is 100 x less than animal Y.
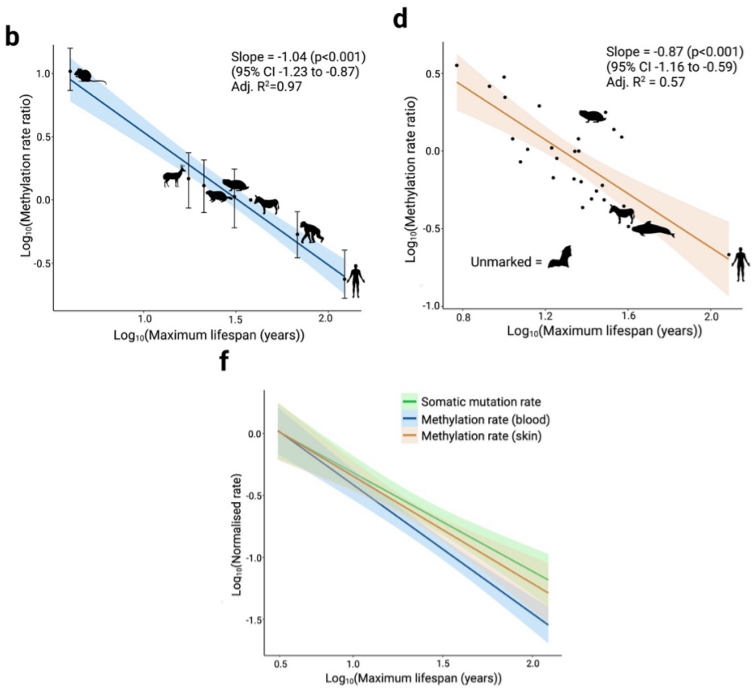
Figure shows a selection of data from Crofts et al. (a) Graph shows the relationship between maximum lifespan and DNA methylation in the blood of different mammalian species. Data is log transformed. (d) Graph shows the relationship between maximum lifespan and DNA methylation in the skin samples of different mammalian species. Data is log transformed. (f) Graph shows the relationship between DNA methylation and maximum lifespan from both blood and skin samples. Additionally, the somatic mutation rate is plotted in relation to maximum lifespan. (Adapted from the preprint under a CC-BY 4.0 International License).
2) The DNA methylation rate and the somatic mutation rate share a similar relationship with maximum lifespan
In a recent study, the rate of somatic mutation (i.e., mutations in cells that are not germline associated, and are therefore not inherited by offspring) was shown to scale proportionally with the lifespan of an organism, i.e., longer living organisms have reduced rates of somatic mutation (3). As this data shows a similar trend to the findings in this study, the authors suggest as one of the possible explanations that there may be a connection between somatic mutations and DNA methylation and, ultimately, an organism’s maximum lifespan.
What could this mean? 1) Aberrant DNA methylation limits maximum lifespan perhaps by affecting the health of the organism. Organisms that live longer may be better at resolving these issues, thereby extending their lifespan and/or, 2) the rates of somatic mutation and DNA methylation are connected by underlying biological processes that are still to be determined.
Why I like this preprint:
The human population is shifting towards a more ‘aged’ population, but we still understand very little about all the complex factors that play roles in the ageing process. Studies like this and many others now suggest that common processes operate across mammals that could contribute to ageing, and moreover, link these processes to certain regulatory events/chemical changes that happen inside our cells. If we could find ways to control these events ourselves, we may find new ways to limit the effects of ageing and age-related diseases on the human body.
Questions for the Authors:
Q1: Have you tried using your model to predict the lifespan of any species?
Q2: The largest organisms in your dataset are humans, but of course human lifespans can now be influenced by additional factors like medical science. Can you control for these effects in your model? What about other longer living species like land tortoises or elephants? Does your model hold true for these organisms?
Q3: Why are most of the skin samples derived from bats? Any why did you select skin samples for this study? Do you have any plans to test other tissue types?
References:
- David Allis, C., & Jenuwein, The Molecular hallmarks of epigenetic control. Nature Review Genetics, 2016.
- Greenberg, M.V.C., & Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nature Reviews Mol. Cell Bio, 2019.
- Cagan et al., Somatic mutation rates scale with lifespan across mammals. Nature, 2022.
doi: https://doi.org/10.1242/prelights.34858
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the genetics category:
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
Loss of MGST1 during fibroblast differentiation enhances vulnerability to oxidative stress in human heart failure
Jeny Jose
A high-coverage genome from a 200,000-year-old Denisovan
AND
A global map for introgressed structural variation and selection in humans
Siddharth Singh
preLists in the genetics category:
SciELO preprints – From 2025 onwards
SciELO has become a cornerstone of open, multilingual scholarly communication across Latin America. Its preprint server, SciELO preprints, is expanding the global reach of preprinted research from the region (for more information, see our interview with Carolina Tanigushi). This preList brings together biological, English language SciELO preprints to help readers discover emerging work from the Global South. By highlighting these preprints in one place, we aim to support visibility, encourage early feedback, and showcase the vibrant research communities contributing to SciELO’s open science ecosystem.
| List by | Carolina Tanigushi |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
September in preprints – Cell biology edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading list. This month, categories include: (1) Cell organelles and organisation, (2) Cell signalling and mechanosensing, (3) Cell metabolism, (4) Cell cycle and division, (5) Cell migration
| List by | Sristilekha Nath et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
June in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell organelles and organisation (2) Cell signaling and mechanosensation (3) Genetics/gene expression (4) Biochemistry (5) Cytoskeleton
| List by | Barbora Knotkova et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
Keystone Symposium – Metabolic and Nutritional Control of Development and Cell Fate
This preList contains preprints discussed during the Metabolic and Nutritional Control of Development and Cell Fate Keystone Symposia. This conference was organized by Lydia Finley and Ralph J. DeBerardinis and held in the Wylie Center and Tupper Manor at Endicott College, Beverly, MA, United States from May 7th to 9th 2025. This meeting marked the first in-person gathering of leading researchers exploring how metabolism influences development, including processes like cell fate, tissue patterning, and organ function, through nutrient availability and metabolic regulation. By integrating modern metabolic tools with genetic and epidemiological insights across model organisms, this event highlighted key mechanisms and identified open questions to advance the emerging field of developmental metabolism.
| List by | Virginia Savy, Martin Estermann |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
March in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) cancer biology 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics and genomics 6) other
| List by | Girish Kale et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
Early 2025 preprints – the genetics & genomics edition
In this community-driven preList, a group of preLighters, with expertise in different areas of genetics and genomics have worked together to create this preprint reading list. Categories include: 1) bioinformatics 2) epigenetics 3) gene regulation 4) genomics 5) transcriptomics
| List by | Chee Kiang Ewe et al. |
January in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell migration 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) genetics/gene expression
| List by | Barbora Knotkova et al. |
End-of-year preprints – the genetics & genomics edition
In this community-driven preList, a group of preLighters, with expertise in different areas of genetics and genomics have worked together to create this preprint reading list. Categories include: 1) genomics 2) bioinformatics 3) gene regulation 4) epigenetics
| List by | Chee Kiang Ewe et al. |
BSDB/GenSoc Spring Meeting 2024
A list of preprints highlighted at the British Society for Developmental Biology and Genetics Society joint Spring meeting 2024 at Warwick, UK.
| List by | Joyce Yu, Katherine Brown |
BSCB-Biochemical Society 2024 Cell Migration meeting
This preList features preprints that were discussed and presented during the BSCB-Biochemical Society 2024 Cell Migration meeting in Birmingham, UK in April 2024. Kindly put together by Sara Morais da Silva, Reviews Editor at Journal of Cell Science.
| List by | Reinier Prosee |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Alumni picks – preLights 5th Birthday
This preList contains preprints that were picked and highlighted by preLights Alumni - an initiative that was set up to mark preLights 5th birthday. More entries will follow throughout February and March 2023.
| List by | Sergio Menchero et al. |
Semmelweis Symposium 2022: 40th anniversary of international medical education at Semmelweis University
This preList contains preprints discussed during the 'Semmelweis Symposium 2022' (7-9 November), organised around the 40th anniversary of international medical education at Semmelweis University covering a wide range of topics.
| List by | Nándor Lipták |
20th “Genetics Workshops in Hungary”, Szeged (25th, September)
In this annual conference, Hungarian geneticists, biochemists and biotechnologists presented their works. Link: http://group.szbk.u-szeged.hu/minikonf/archive/prg2021.pdf
| List by | Nándor Lipták |
2nd Conference of the Visegrád Group Society for Developmental Biology
Preprints from the 2nd Conference of the Visegrád Group Society for Developmental Biology (2-5 September, 2021, Szeged, Hungary)
| List by | Nándor Lipták |
EMBL Conference: From functional genomics to systems biology
Preprints presented at the virtual EMBL conference "from functional genomics and systems biology", 16-19 November 2020
| List by | Jesus Victorino |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
ECFG15 – Fungal biology
Preprints presented at 15th European Conference on Fungal Genetics 17-20 February 2020 Rome
| List by | Hiral Shah |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Zebrafish immunology
A compilation of cutting-edge research that uses the zebrafish as a model system to elucidate novel immunological mechanisms in health and disease.
| List by | Shikha Nayar |











 (No Ratings Yet)
(No Ratings Yet)