Sensory input drives rapid homeostatic scaling of the axon initial segment in mouse barrel cortex
Posted on: 10 April 2020
Preprint posted on 28 February 2020
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-020-20232-x
And yet it moves! Sensory input drives AIS changes in length in the mouse barrel cortex
Selected by Ana Dorrego-RivasCategories: neuroscience
Background and introduction
In most neurons, the axon initial segment (AIS) is the site for action potential generation and tuning, due to its high concentration of voltage-gated channels. While the AIS has a strong molecular network, multiple studies revealed an activity-dependent structural plasticity to maintain neuronal homeostasis. In cultured neurons, the AIS can change in position as a response to chronic depolarization. However, these changes in position and/or length seem to vary within neuronal type, making AIS plasticity a more complex matter.
In vivo, AIS structural plasticity can happen during development, like in the virtual cortex, but also in the context of sensory deprivation: the AIS from neurons of the nucleus magnocellularis changes in length when facing a lack of auditory stimuli. Still, the current knowledge we have about AIS remodeling and how it translates to maintain neuronal excitability in the brain circuits is limited. In this study, the authors use the mouse whisker-to-barrel system, a very well-known studied sensory pathway, to study AIS plasticity in a behaviorally relevant context.
Key findings
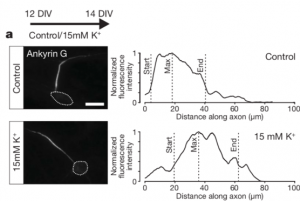
The authors first sought to monitor the length of the AIS of pyramidal neurons in layers II/III and V of the primary somatosensory cortex (SF1B) within different developmental timepoints. For that, they used immunolabelling on brain slices for ßIV-spectrin, one of the key molecules of the AIS, and found the structure to be particularly dynamic: the AIS length increased notably between the beginning and the second week of the postnatal period and then shortened to keep an average length. This AIS lengthening was followed by an overall increase of AnkyrinG (AnkG), the master organizer of the AIS, in all of its isoforms.
Since the period of AIS length reduction coincides with the onset of active whisking behavior, the authors hypothesized that this activity is the one modulating the AIS structural maturation. To test it, they focused on a sensory-deprivation strategy consisting in trimming the whiskers at the moment of birth and observe the AIS structure after 15, 21 and 45 days. Neurons from layers II/III displayed a lengthening of the AIS in all the tested conditions as a response to the deprivation, but those from layer V remained unaffected. To assess if this scenario was reversible with a recovery of the sensory input, the authors trimmed the whiskers of the mouse, let them grow back and then assessed AIS structure: the length went back to mature levels. Interestingly, when whisker trimming was performed in older animals, the AIS also increased in length in the same layers. These striking results are a proof that AIS plasticity at the primary somatosensory cortex is sensory-input dependent even in the adult brain!
But, how do these structural changes translate to the excitability of the neurons? The authors performed patch-clamp recordings in layer II/III neurons in control and sensory-deprived mice. After confirming no changes in the resting membrane potential, they found that the action potential firing rates were higher for the mice with shorter whiskers and that the current they needed to inject to fire an action potential was lower. After performing a post-hoc staining of the recorded neurons, the authors confirmed a correlation between the length of the AIS and the increased intrinsic excitability of layer II/III neurons.
The data confirms a reduction of the AIS length with a progressive recovery of the sensory input within hours, however, is it possible to induce a faster re-structuration? To answer this, the authors used an enriched environment (EE) where the mice were placed for different timings — 1h, 3h and 6h. This time, whiskers were trimmed unilaterally (and not bilaterally as the previous experiments), implying that that the SF1B contralateral to the intact whisker side (here referred to as “EE”) received higher activation by the whisker-to-barrel pathway than the ipsilateral side, which represents the control. After confirming the activation of the network through c-fos staining, the authors observed a decrease in length of the AIS in the EE neurons in all the measured timepoints. The exposition of the animals to a sensory enriched environment triggers a faster AIS relocation! Notably, when the mice were placed in the EE for 3h and put back for other 3h at the basal environment, the AIS remained unchanged. Again, this was true for neurons from layers II/III but not for those from layer V. Altogether, these powerful results reveal a very quick and adaptive AIS behavior to different sensory inputs.
To assess the impact of these fast changes in neuronal excitability, the authors performed electrophysiology recordings in control and EE neurons of layers II/III. In line with the findings above, the EE neurons displayed a higher threshold and frequency of firing action potentials versus the control, revealing an overall reduced intrinsic excitability.
In conclusion, this study makes a step forward on understanding AIS plasticity in vivo and, more specifically, within a concrete behavior context. It will definitely be the start of further studies on how these excitability changes integrate in the whole network and, therefore, in the brain.
Why did I choose this article?
I am a huge passionate about the AIS and the plasticity field and I appreciate the high quality of this work. Not a lot is known about the AIS role on maintaining neuronal homeostasis in vivo, and this study covers a big part of it. Some studies were performed on the auditory system, but this one is pioneer on this kind of sensory information (tactile) and the whisker-to-barrel pathway.
The way the article is written, first addressing the structural changes and then the functional ones, makes it easier and enjoyable to read. It is also based on scientific statements provided by numerous repetitions of experiments and trustable statistics. Scientific robustness is a must for all the researchers. I also found the discussion very complete, covering all the aspects to which the data did not have the exact answer.
References
Evans, M.D., Dumitrescu, A.S., Kruijssen, D.L., Taylor, S.E., and Grubb, M.S. (2015). Rapid Modulation of Axon Initial Segment Length Influences Repetitive Spike Firing. Cell Rep 13, 1233-1245.
Galiano, M.R., Jha, S., Ho, T.S., Zhang, C., Ogawa, Y., Chang, K.J., Stankewich, M.C., Mohler, P.J., and Rasband, M.N. (2012). A Distal Axonal Cytoskeleton Forms an Intra- Axonal Boundary that Controls Axon Initial Segment Assembly. Cell 149, 1125-1139.
Grubb, M.S., and Burrone, J. (2010). Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070-1074.
Gutzmann, A., Ergul, N., Grossmann, R., Schultz, C., Wahle, P., and Engelhardt, M. (2014). A period of structural plasticity at the axon initial segment in developing visual cortex. Front Neuroanat 8, 11.
Kuba, H., Oichi, Y., and Ohmori, H. (2010). Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 465, 1075-1078.
doi: https://doi.org/10.1242/prelights.18432
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
Electrophysiological correlates of conscious experiences during sleep: Lucid dreams, sleep paralysis, out-of-body experiences, and false awakenings
uMontreal Neuro preLighters et al.
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (2 votes)
(2 votes)