Synergistic olfactory processing for social plasticity in desert locusts
Posted on: 8 April 2024 , updated on: 10 January 2025
Preprint posted on 15 September 2023
Article now published in Nature Communications at http://dx.doi.org/10.1038/s41467-024-49719-7
The smell of a movable feast: researchers investigate the neuronal basis of olfactory-mediated foraging behavior in locusts.
Selected by T. W. Schwanitz, Lukas WeissCategories: neuroscience
Updated 9 January 2025 with a postLight by Timothy W. Schwanitz and Lukas Weiss
This was an interesting and thorough study to begin with, so not much has changed in the final published version of the article. The most striking changes between the final article and the preprint lie in the analysis: in figure 5, we now see only 6 motifs characterizing odor response activity, and the results do seem different from 5 Cii in the preprint. This part of the paper will be of the greatest interest for anyone trying to make sense of calcium imaging data. These data are inherently tricky. How the authors try to tackle them initially and then revise their analysis to extract useful information from the data no doubt holds valuable lessons for the field.
In broad brushstrokes, most of the changes revolve around reformatting figures. The figures in the final article have been polished to have fewer graphics in several panels. While this does indeed come across as cleaner, readers who are especially interested in this work should compare with the preprint. For example, I found the bliss scores portrayed in figure 4 of the preprint interesting and was sad to see them go. A few of the removed panels have been saved in the supplement, which I can also recommend scrolling through for a fuller picture of the work, as supplementary analyses have also been added, e.g., some interesting EAG studies.
Introduction
Locusts were one of the Biblical plagues of Egypt—since the advent of human agriculture, fear of these insects and their voracious appetites has echoed throughout religion and storytelling. They have the ability to aggregate into dense clouds that destroy whole harvests. In spite of our ancient enmity with these insects, however, it is only recently that we’ve begun to understand how and why they form such imposing swarms.
Locusts (in this study, Schistocerca gregaria) come in two forms: a solitary and a gregarious form. Solitary locusts are bright green in color and do not aggregate. Gregarious locusts, by contrast, have a bold yellow-black appearance and do form large swarms. Locusts that are reared in high densities become gregarious, while those reared at low densities become solitary. Little is known about differences in sensory abilities between these two forms.
Petelski, Günzel and colleagues wanted to understand the differences between these two locust phenotypes and in particular the way in which they both process odors in the brain. To do so, they turned to calcium imaging, a technique that makes it possible to visualize neuronal activity by adding proteins that fluoresce in the presence of calcium (which flows into a neuron when it fires). Compared with fruit flies, calcium imaging in the locust smell-processing center, the antennal lobe, is especially tricky: in fruit flies, neurons that respond to a given odor converge on a single spherical bundle of synapses called a glomerulus. Fruit flies have 51 of these olfactory glomeruli (Bates et al., 2020). By contrast, the glomeruli in locusts are organized in a radial pattern made up of over 1,000 microglomeruli (Fig. 2C).
The authors first established that odor plays a role in the aggregation of gregarious locusts; then, they observed gregarious and solitary locust brain activity when exposed to various biologically relevant odors. Finally, they attempted to wrangle sense out of the 1,000 plus little blobs of activity that came out of their calcium imaging data.
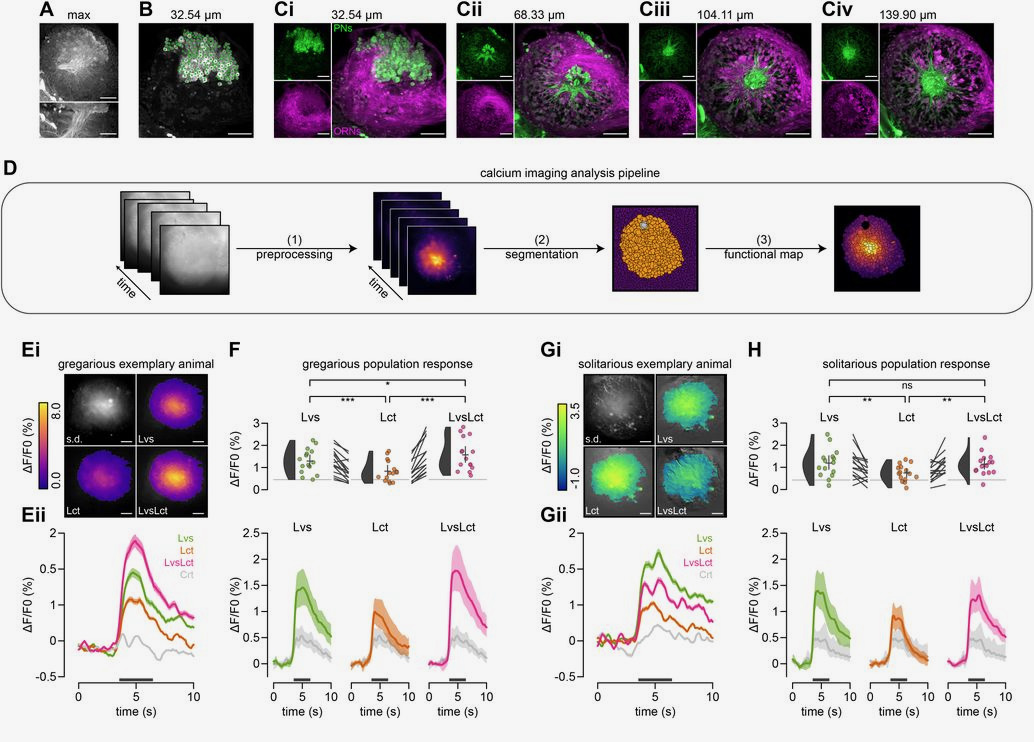
Fig. 2: calcium imaging of the locust antennal lobe. A-Civ Images of the locust antennal lobe, with Ci-iv showing different Z-stacks of projection neurons in green and olfactory sensory neurons. D shows an overview of the authors’ analysis pipeline. E-Gii show calcium image traces from gregarious animals on the left and solitary animals on the right.
Highlighted results
To establish that olfaction plays a more prominent role in gregarious locust behavior, the authors first conducted a behavioral assay where a single locust in an arena could freely choose between going to a container with leaves, a container with locusts, or a container with both. Importantly, these containers could have holes to allow the scent to dissipate, be transparent to let the trial locusts see its compatriots, or both. The authors found that scent played an especially prominent role in the decision-making process of the gregarious locusts. Using a GC-MS analysis of the leaves coupled with calcium imaging, the authors concluded that leaf alcohol acetate is the primary odor driving locust attraction to leaves and produces similar calcium traces to whole leaf odor.
Petelski, Günzel and colleagues then used calcium dye backfills to image the olfactory projection neurons, capturing activity in the dendrites and somata when exposed to leaf alcohol acetate odor, locust odor, or both odors together, resulting in few key conclusions:
- Calcium imaging responses were consistent across trials within the same animal and across several animals. Individual response units (called granules in the paper) responded in a stable combinatorial way to the applied odors. Olfactory responses appear to function in a combinatorial code in locusts.
- Gregarious, but not solitary animals, showed higher calcium responses to the food cues (leaf/leaf alcohol acetate) when the social odor cue (locust smell) was in the mix. Gregarious animals have a higher proportion of mixture-specific olfactory units than solitary animals. Gregarious locusts respond to the coincidence of both social and food odors.
- The responses of projection neuron somata can be divided into different response motifs. An analysis of these motifs shows that there are more synergistic interactions in gregarious locusts. Projection neuron somata show a higher degree of response overlap and integration between social and food odor in gregarious locusts.
- The authors created a model that has 92% accuracy in determining if a locust was solitary or gregarious based on their projection neuron somata response motifs. Projection neuron response motifs can predict the locust phenotype.
Taken together, these results suggest that changes in locust gregarious olfactory responses versus solitary locust olfactory responses are due to a subtle reweighting of existing neuronal pathways and dynamics. The interaction between social and food odor encoding in the antennal lobe could play a role in shaping collective foraging decisions in locusts. Teasing apart these subtle interactions in locusts and in other insects will no doubt be enriching for the broader field of sensory processing.
Why we like this study
Calcium imaging is a challenging technique: preparations require a lot of effort, limiting sample sizes and magnifying the impacts of noise on the data. We therefore appreciated the authors’ attempts to wrangle meaning out of a tricky dataset, further complicated by the biological structure of the locust antennal lobe. We especially liked seeing the inclusion of an insect with a different antennal lobe Bauplan than many of the more commonly imaged insects.
doi: https://doi.org/10.1242/prelights.37050
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)