The unique synaptic circuitry of specialized olfactory glomeruli in Drosophila melanogaster
Posted on: 16 July 2023 , updated on: 21 July 2023
Preprint posted on 25 April 2023
Ever tried untangling a plate of pasta? This preprint tries to make sense of the spaghetti-like connections of narrowly and broadly tuned olfactory glomeruli in the Drosophila brain.
Selected by T. W. SchwanitzCategories: animal behavior and cognition, neuroscience
Introduction to untangling pasta (neural connectomics)
Neuroscience borrows a lot of terminology from electrical engineering, like “circuit motifs” and “wiring diagrams.” Words like these suggest straight lines—with inputs connecting to outputs in an orderly manner. But in reality, terms like “pasta plate” or “spaghetti diagram” would be more apt for describing how neurons are connected in the brain of living organisms. The brain is wetware, not hardware. A recent study by Gruber and colleagues uses laser branding and electron microscopy to try to make sense of this welter of living wires. The authors investigated the structure of the olfactory regions in the Drosophila melanogaster brain that respond to specific odors, as compared with those that respond to many odors.
In insects (and similarly in vertebrates), olfactory sensory neurons on the antennae converge in a region called the antennal lobe. Within this region, neurons detecting a particular odorant or group of odorants form spherical clusters known as glomeruli, which literally means “little balls of thread.” Projection neurons transmit information from glomeruli to higher brain centers. Local interneurons connect glomeruli together, allowing them to modulate each other’s activity.
That was the simple wiring diagram description. Now for the details that make this more like a spaghetti plate: neurons can synapse onto themselves; most neuronal outputs connect to multiple inputs (termed polyadic synapses, see Fig. 1 of the preprint); dendrites responsible for input processing also have outputs (dendro-dendritic synapses); and, finally, a whole smorgasbord of neurotransmitters and neuromodulators add additional subtlety and complexity—akin to the seasoning on pasta.
Gruber and colleagues wondered if glomeruli that respond to only a few odors have common features that differ from glomeruli that respond to many odors. They used focused ion beam-scanning electron microscopy to investigate the DA2 glomerulus, which is narrowly tuned and responds to the highly aversive odorant geosmin, and the DL5 glomerulus, which is broadly tuned to a number of aversive odorants such as benzaldehyde (I keep them straight by remembering that the one with the bigger number responds to more odorants). The researchers added VA1v to their comparison, a narrowly tuned glomerulus that responds to the aggregation signal methyl laurate. The scientists took images at 20 nanometer distance intervals (unimaginably small steps) via focused ion beam-scanning electron microscopy, and they then reconstructed 3D volumes via computer software. To keep track of the location of their glomeruli of interest, the authors used a two-photon laser to brand lines in the brain around the glomeruli—thereby making it easier to find the edges of the glomeruli in all those virtual slices.
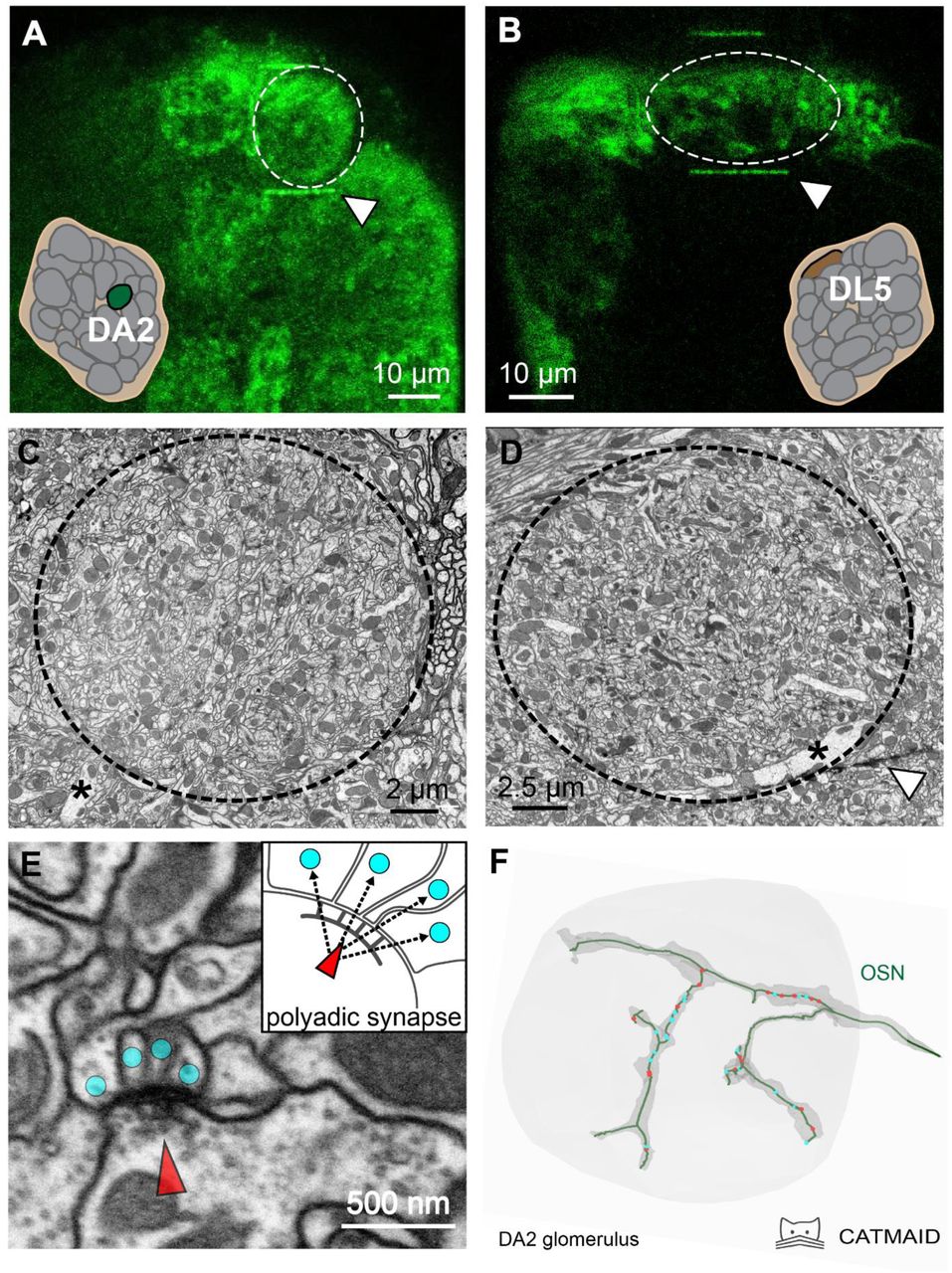
Fig. 1. Overview of the glomerular structure. A) and B) both show the two main glomeruli investigated in this study via in vivo functional imaging. The thin green line above and below both glomeruli is the laser branding mark. C) and D) show these glomeruli as scanning electron microscope images. In D), the white triangle in the bottom right points to a thin black line that corresponds to the laser branding mark. E) shows an example of a polyadic synapse (a tetrad in this case), both as a scanning electron microscope image and as a diagram. F) shows a digital reconstruction of an olfactory sensory neuron based on the electron microscope images.
Highlighted results of the study
This study finds a number of interesting patterns that could lay the groundwork for future hypotheses. The authors distill their comparison of broadly tuned versus narrowly tuned glomeruli into five main findings:
- Narrowly tuned glomeruli have stronger feedforward olfactory sensory neuron connections to both uniglomerular projection neurons and multiglomerular neurons. That is to say, the input neurons have more connections to output neurons and interneurons.
- Narrowly tuned glomeruli have much stronger axo-axonic communication between sister olfactory sensory neurons. In other words, the input neurons have more possibilities to modulate each other’s input.
- Narrowly tuned glomeruli have stronger dendro-dendritic connections between uniglomerular projection neuron dendrites. The output neurons are better able to synchronize with each other and modulate each other.
- Narrowly tuned glomeruli have less feedback from uniglomerular projection neurons to multiglomerular neurons, a group that includes both multiglomerular projection neurons and local interneurons. The output neurons do not have as many opportunities to modulate the neurons that communicate between glomeruli.
- Narrowly tuned glomeruli have less feedback from multiglomerular neurons to olfactory sensory neurons. There are fewer opportunities for interneurons and multiglomerular projection neurons to modulate the input neurons, i.e., the inputs are subject to less “cross-talk” from other glomeruli.
These findings support the notion that narrowly tuned glomeruli establish more direct links with higher brain centers, with inputs being less influenced by other glomeruli. Conversely, broadly tuned glomeruli seem more receptive to signals from neighboring glomeruli. Narrowly tuned glomeruli exhibit greater intraglomerular modulation of inputs and outputs, potentially amplifying and synchronizing signal transmission.
One final finding that stands out: autapses, or synapses of a neuron onto itself, occurred at a greater frequency than the authors expected. This finding was especially true for the one uniglomerular projection neuron that leaves the broadly tuned DL5 glomerulus. It appears that the large, sprawling dendrite of this neuron synapses onto itself such that distant regions are more interconnected—perhaps providing the possibility for synchronizing the activity of the neuron.
Why I liked this preprint
As big, whole-brain connectomes become increasingly feasible, the methods used in this study for quickly making connectomes of important regions become especially interesting—they could make it possible to compare select regions across multiple individuals, to see just how representative one connectome can be. Moreover, because these methods link connectomics to functional work, it could be possible to compare between individuals that respond differently to a given stimulus and see how the physical connectivity of the brain influences different responses. Finally, this study provides a lot of interesting basic information about how important olfactory pathways are set up.
Questions for the authors
- In the study, you use the category “multiglomerular neurons” for both local interneurons and multiglomerular projection neurons. Could you elaborate on why you had to put these two disparate types of neurons in the same category, while you were able to differentiate olfactory sensory neurons and uniglomerular projection neurons? What additional steps would it take differentiate these?
- Do you have any reason to think that there might be similar patterns of differences in the multiglomerular projection neurons as with the uniglomerular projection neurons between broadly and narrowly tuned glomeruli?
- Do you think your method could complement whole connectomes of a single brain by comparing a region of interest in several individuals, or do you see this method as opening up other avenues of research?
- In your workflow, which parts of the process actually take the most time and are the main bottleneck? The tracing perhaps?
- In Figure 2 – figure supplement 1, it looks like there are some synapses without connections (no postsynaptic contacts per T-bar). Could you elaborate on that finding? Are those synapses in the process of forming/being pruned, or could they be the product of damaged brain slices?
doi: https://doi.org/10.1242/prelights.35133
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)