Robotic microscopy for everyone: the OpenFlexure Microscope
Posted on: 27 March 2020 , updated on: 2 May 2020
Preprint posted on 3 December 2019
Article now published in Biomedical Optics Express at http://dx.doi.org/10.1364/BOE.385729
Categories: bioengineering
Background
Microscopes are an essential tool for clinical applications including diagnosis of infectious pathogens in endemic areas, and for scientific analysis in basic biology and physics labs. However, in much of the world, access to microscopy is limited by the cost of acquisition and maintenance of the imaging equipment. Moreover, in resource-limited settings, the chain of supply of parts that might need repair or replacement might not be as easily available, leading to high-end microscopes being out of service for long times until maintenance can take place. Open-source hardware has the potential to revolutionise the distribution of scientific instrumentation, impacting research in multiple ways, as well as local manufacturing, and education. To this end, in multiple contexts including research and clinics, 3D printers have become increasingly available. As a direct result of this, 3D printing has become a useful platform for prototyping and manufacturing laboratory devices. In their preprint, Collins et al (1) present the OpenFlexure Microscope design, a 3D printed automated microscope capable of motorised sample positioning and focus control (Figure 1).
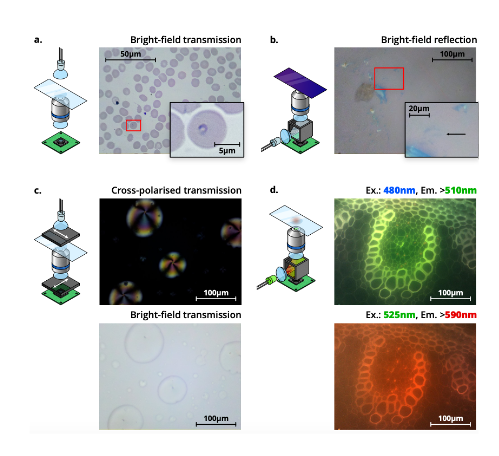
Key findings and developments
General development
- The OpenFlexure Microscope is a 3D-printed and fully-automated laboratory microscope, which has a number of options readily available including trans- and epi-illumination, polarisation contrast imaging, and epi-fluorescence imaging.
- The OpenFlexure Microscope has been designed to enable low-volume manufacturing and maintenance by local personnel.
- It is capable of providing precise 3D motion for focus and sample positioning, with a range of motion suitable for a wide range of research applications.
- The OFM can be constructed with a range of different interchangeable optics modules, allowing use of different cameras and lenses depending on the application.
- The design has been trialled in multiple countries around the world, and has been already distributed for implementation in Tanzania and Kenya for educational purposes and clinical applications in field settings.
Specifics points about OFM
Imaging modes
- The imaging modes of the microscope are the following:
- Bright field trans-illumination: this is the standard mode of the OFM. Light passing through the sample is imaged with an RMS objective, a tube lens, and an 8MP CMOS sensor. This setup has been tested for malaria diagnosis, and allowed the automatic acquisition of images.
- Bright field epi-illumination: This is possible by the insertion of a 50/50 beam splitter within a printed filter cube between the tube lens and the sensor. Illumination is provided by a collimated diffused LED reflected through the objective by the beam-splitter. The authors tested this setup for the detection of graphene flakes.
- Polarisation-contrast imaging: The OFM can be used for polarisation-contrast imaging by placing a linear polariser between the illumination and the sample, and an orthogonal polariser between the tube lens and the sensor.
- The OFM can perform low-cost fluorescence microscopy by inserting a dichroic beam splitter and optical filters within a printed filter cube between the tube lens and the sensor, and illuminating with an LED of the desired excitation wavelength. By selecting appropriate filters and LEDs, illumination modules can be constructed for any fluorescence wavelength.
- Calibrating the intensity response of the Raspberry Pi camera module (4) is an important consideration for making the OFM usable.
- The authors provide estimated parts costs for various configurations of the microscope.
Automated imaging
- Autofocus is crucial for automated microscopy. The OFM’s software includes two image-based autofocus algorithms, capable of automatically bringing a thin, flat sample into sharp focus. The first option makes use of a Laplacian filter. The second option is to measure sharpness while moving the stage continuously, monitoring the size of each frame in the MJPEG video stream.
- Two key applications of automated microscopy are tile scanning and time-lapse imaging, which the OFM is capable of doing.
Software and usability
- For OFM use, OpenFlexure eV, is a cross-platform graphical application that enables basic functionality, and allows user-interfaces for more complex plugins. Programming experience is not required.
- The server software is distributed as a pre-built SD card image for a Raspberry Pi microcomputer, and is common to all OFMs.
- Developers are able to create new plugins specific to their needs, and enable them on a per-microscope basis.
Manufacturing and sustainability
- A key aim of the OpenFlexure project is to enable local production. Various rounds of optimization have resulted in the OFM being easy to print, assemble, and source parts for. This ease of production allows customisation and maintenance of the equipment without external service engineers.
- The OFM has been engineered to print without support material. This makes the design both easier and faster to print, and avoids the risk of damaging printed parts while removing support.
- Non-printed parts have been carefully considered to balance cost, performance, and ease of sourcing. Some components have been carefully chosen to improve the microscope’s lifetime, while broken parts can be quickly and easily printed locally and replaced with minimal downtime.
What I like about this preprint
I am a big supporter of open access and open science. I like that this work addresses a real need across laboratories worldwide. We often see discussed the cost of microscopes, but not many setups consider the cost of maintenance, replacement and supply chains in resource-poor settings. I think in their full design, the authors kept this in mind, and ensured reproducibility in multiple countries to ensure the aim of the microscope is achieved. Moreover, I like that they use a relatively recent technology for very helpful uses in public health, education and research.
Open questions
*Note: all questions with answers can be found at the end of this page.
- I think your setup is an excellent idea. Given the widespread distribution that OFM was created for, how will you train users on OFM use, including the hardware and software for image analysis both by beginners and more advanced users capable of creating plugins for their own needs, in resource-poor settings?
- Have you considered, together with designers of other open source microscopes (eg. Octopi (2), OptiJ (3)), unifying a pipeline of distribution and training so as to benefit the most people possible in the use of microscopy in resource-poor settings?
- 3D printing is indeed beginning to revolutionize diagnosis and medicine in fronts such as prosthesis design. It would be a great asset if 3D printers were widely available in all regions. How will you ensure the widespread distribution of the 3D printed microscopes, if 3D printers are not always available locally? Is it an idea to integrate in the same program addressing public health, the concept and potential usefulness of 3D printers in clinical settings (including the benefit to diagnosis via microscope production)?
References
- Collins, J.T. et al, Robotic microscopy for everyone: The OpenFlexure Microscope, bioRxiv, 2019. doi:10.1101/861856
- Li, H. et al, Octopi: Open configurable high-throughput imaging platform for infectious disease diagnosis in the field, bioRxiv, 2019. doi:10.1101/684423
- Vallejo Ramirez P.P. et al, OptiJ: Open-source optical projection tomography of large organ samples, Sci Rep, 2019. 9(1):15693, doi:10.1038/s41598-019-52065-0.
- Bowman, R., et al, Flat-field and colour correction for the Raspberri Pi camera module, arXiv, 2019. arXiv:1911.13295.
Acknowledgement
Thank you to Richard Bowman and Joel Collins for their engagement and answering the open questions.
doi: https://doi.org/10.1242/prelights.17955
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the bioengineering category:
A Novel Chimeric Antigen Receptor (CAR) - Strategy to Target EGFRVIII-Mutated Glioblastoma Cells via Macrophages
Dina Kabbara
Human pluripotent stem cell-derived macrophages modify development of human kidney organoids
Theodora Stougiannou
Matrix viscoelasticity regulates dendritic cell migration and immune priming
Roberto Amadio
preLists in the bioengineering category:
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
EMBL Synthetic Morphogenesis: From Gene Circuits to Tissue Architecture (2021)
A list of preprints mentioned at the #EESmorphoG virtual meeting in 2021.
| List by | Alex Eve |
3D Gastruloids
A curated list of preprints related to Gastruloids (in vitro models of early development obtained by 3D aggregation of embryonic cells). Updated until July 2021.
| List by | Paul Gerald L. Sanchez and Stefano Vianello |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
EMBL Seeing is Believing – Imaging the Molecular Processes of Life
Preprints discussed at the 2019 edition of Seeing is Believing, at EMBL Heidelberg from the 9th-12th October 2019
| List by | Dey Lab |
Lung Disease and Regeneration
This preprint list compiles highlights from the field of lung biology.
| List by | Rob Hynds |
Advances in microscopy
This preList highlights exciting unpublished preprint articles describing advances in microscopy with a focus on light-sheet microscopy.
| List by | Stephan Daetwyler |











 (No Ratings Yet)
(No Ratings Yet)