Neuronal activity drives pathway-specific depolarization of astrocyte distal processes
Posted on: 20 July 2021 , updated on: 21 July 2021
Preprint posted on 4 July 2021
Eavesdropping neuron-astrocyte conversations: the Dulla lab unravels a novel form of neuron-astrocyte crosstalk using genetically encoded voltage imaging
Selected by Kristina KuhbandnerCategories: neuroscience
Background
Electrical signaling plays a central role in the transmission of information between neurons. How neurons generate and propagate these signals has been extensively studied in the past. Importantly, to fulfil their duties, neuronal cells also need the support of other cells such as oligodendrocytes and astrocytes. Astrocytes physically and functionally interact with neuronal synapses, and play a role in regulating extracellular levels of potassium (K+) and the neurotransmitter glutamate, thereby controlling excitatory neurotransmission. For example, the clearance of glutamate is facilitated by excitatory amino acid transporters (EAATs) located on distal astrocyte processes (DAPs). These transporters are known to be voltage-dependent and can be inhibited upon neuronal activity. Importantly, until now it has been assumed that astrocytes themselves are not electrically active, because following neuronal activity only minimal membrane potential changes were observed at the soma of astrocytes (1). To explain this discrepancy, Armbruster and colleagues hypothesized that local depolarization at distal processes might be responsible for the activity-induced inhibition of EAATs.
To investigate whether DAPs undergo electrical changes that can’t be detected in the soma, they specifically expressed the genetically encoded voltage indicators (GEVIs) Archon1 or Arclight in mouse cortical astrocytes (2, 3). These membrane-bound fluorescent sensors report membrane voltage dynamics as changes in fluorescent signal intensities.
Main findings
1. Astrocytes show fast, activity-dependent depolarization
To specifically express the GEVIs Archon1 or Arclight in astrocytes, the authors employed AAV-mediated transduction under the control of a modified GFAP promoter. After electrical stimulation of ascending axons in acute brain slices, confocal images were taken to measure changes in fluorescence intensities to investigate activity-induced membrane potential changes in astrocytes. Both, Archon1 and Arclight exhibited stimulus-evoked changes in fluorescence (F/F0) consistent with astrocyte depolarization (Fig. 1A, B). Of note, depolarization time derived from analysis of GEVI F/F0 was approximately five times faster than depolarization time measured in the soma by whole cell patch clamping.
2. Activity-induced astrocyte depolarization is locally restricted, stable and pathway- specific
To specifically determine depolarized regions in astrocytes, principal component analysis/independent component analysis was performed. In the identified “hotspots” of astrocyte depolarization, stimulus-evoked fluorescent changes were significantly enhanced and stable over repeated trials. Then, Armbruster et al. compared fluorescence changes in hotspots after either stimulating ascending cortical or Layer II/III intracortical axons (Fig. 1C). Interestingly, there was only minimal spatial overlap of depolarized hotspots evoked by stimulation of either ascending or intracortical axons, indicating that stimulus-dependent depolarization of astrocytes is pathway-specific (Fig. 1 D).

Figure 1: Fast, activity-dependent and pathway-specific depolarization of astrocytes. (A) Archon1 and (B) Arclight exhibit progressive depolarization with increasing stimuli number (x-axis). (C) Illustration of the experimental setting to study pathway-specific depolarization using ascending (red) or intracortical (blue) stimulators. (D) Example ROI maps of Arclight-expressing astrocytes after stimulating either ascending or intracortical axons (modified after Armbruster et al., 2021, Fig.1 and Fig.3)
3. Astrocyte depolarization is regulated by EAAT activity and presynaptic K+ release
Next, the authors wanted to investigate how neuronal activity causes depolarization of DAPs. Therefore, they manipulated different factors known to be involved in neuron-astrocyte crosstalk. Fluorescent signals were abrogated after using Tetrodotoxin to block voltage-gated sodium channels, verifying that neuronal activity is required for astrocyte depolarization. Inhibition of glutamate uptake by EAATs only caused a partial reduction of GEVI fluorescent changes, indicating that additional mechanisms may contribute to the regulation of astrocyte depolarization (Fig. 2A). Subsequently, the expression of the astrocytic voltage-dependent K+ channel Kir4.1, which buffers increased extracellular K+ concentrations following neuronal activity, was manipulated. While Kir4.1 overexpression significantly reduced depolarization, its inhibition resulted in increased depolarization (Fig. 2 B). These findings suggest that Kir4.1-mediated K+ uptake helps to regulate activity-dependent depolarization and that the increase in extracellular K+ levels is the main trigger for DAP depolarization.
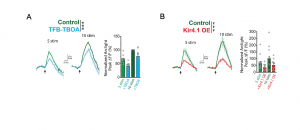
Figure 2. EAAT activity and increased extracellular K+ levels contribute to astrocyte depolarization. Reduced depolarization following (A) application of the EAAT-inhibitor TFB-TBOA or (B) overexpression (OE) of Kir4.1 (modified after Armbruster et al., Fig. 5).
4. DAP depolarization affects the clearance of glutamate
Finally, the authors investigated whether activity-induced depolarization of DAPs modulates astrocyte function, e.g. glutamate uptake. It has been shown that glutamate clearance by EAATs is inhibited by depolarization and slowed down by neuronal activity.
To visualize glutamate clearing after manipulation of activity-dependent depolarization, the fluorescent glutamate reporter iGluSnFr was used. When depolarization was decreased by Kir4.1-overexpression, the slowing of glutamate clearance was reduced. Contrarily, enhancing depolarization by blocking Kir4.1 increased the activity-dependent slowing of glutamate clearance. This demonstrates that EAAT function is inhibited by activity-dependent depolarization of DAPs.
Why I like this preprint
For a long time, neuroscience research mainly focused on neurons. Only in the last decades, other cells in the central nervous system have gained more attention and their critical role in neurological functions has been unraveled. However, their exact contributions remain a mystery. In this study, Armbruster et al. use an elegant approach to investigate the electrical response of astrocytes to neuronal activity. Their findings challenge the prior assumption that astrocytes are not electrically active and provide a method to further decipher the language of neuron-astrocyte communication. Ultimately, this will foster our knowledge about astrocyte-neuron interactions and their implications in health and disease.
Questions to the authors
- The present experiments are performed under “normal” conditions. However, during neuroinflammatory processes and reactive astrocytosis, DAP properties might be altered. Are you planning to investigate DAP depolarization also in reactive astrocytes, for example after application of LPS?
- Do you think a disruption in this form of astrocyte-neuron communication might be involved in the pathophysiology of neurological diseases such as schizophrenia, which is associated with glutamatergic dysregulation?
References
- Zhou et al., Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95,134-43 (2006).
- D. Piatkevich et al., A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat Chem Biol 14, 352-360 (2018).
- Jin et al., Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 75, 779-785 (2012).
doi: https://doi.org/10.1242/prelights.30104
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
Kosmos: An AI Scientist for Autonomous Discovery
Roberto Amadio et al.
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (No Ratings Yet)
(No Ratings Yet)