Brain cooling marginally increases maximum thermal tolerance in Atlantic cod
Posted on: 24 June 2019
Preprint posted on 3 June 2019
Article now published in The Journal of Experimental Biology at http://dx.doi.org/10.1242/jeb.208249
Cool-headed fish survive in hot places – Jutfelt et al. mount cooling plates on Atlantic cod heads and find the loss of equilibrium at thermal extremes may be linked to compromised brain nerve function.
Selected by Rasmus ErnCategories: ecology, physiology
Background
- The critical thermal maximum (CTMAX) is the temperature where fish exhibit loss of equilibrium (LOE) because of a temperature-induced collapse of vital physiological functions. CTMAX defines the upper limit of a species’ fundamental thermal niche and has been applied widely in studies on the responses of fishes to ocean warming (Sunday et al., 2012). Identifying the physiological functions underlying the LOE at CTMAX is therefore highly relevant for understanding how anthropogenic climate change will affect aquatic ecosystems.
- The oxygen-limitation hypothesis, described in the concept of oxygen- and capacity-limited thermal tolerance (reviewed in Pörtner, 2010), posits a temperature-induced collapse of the cardiovascular system and insufficient tissue oxygen supply for vital physiological functions as the central mechanism underlying the LOE in fishes at CTMAX. However, recent studies find several fish species maintain cardiovascular performance at thermal extremes (reviewed in Jutfelt et al., 2018), indicating that the thermal tolerance of other vital functions must be responsible for their LOE at CTMAX.
- Early literature suggests that compromised brain nerve function at thermal extremes is the mechanism underlying the LOE in fishes at CTMAX (Friedlander et al., 1976). To test this hypothesis, Jutfelt et al. mounted cooling plates on the head of Atlantic cod (Gadus morhua) and assessed if localised cooling of the brain increased the CTMAX of this species.
Methods
- Custom-built aluminium brain coolers (Fig. 1A) were attached to the top of the head of the cod using cyanoacrylate glue and silk sutures (Fig. 1B) and connected to a thin flexible silicone tubing that allowed water to be flushed through the coolers to control their temperature (Fig. 1C).
- Tests on terminally anesthetised fish with thermocouples in the brain showed the cooling capacity of the brain coolers on brain tissue in different brain regions was 2–6°C.
- CTMAX was assessed in three groups of fish: 1) a control group without brain-coolers, 2) a group with brain-coolers supplied with ambient ramping-temperature water, and 3) a group with brain-coolers supplied with ice-cold water.
- The CTMAX trials were performed in aquaria with fully aerated water using a temperature ramping rate of 10°C h−1. Fish were deemed to have reached their CTMAX at the temperature where they exhibited LOE and were unable to right themselves within three seconds.
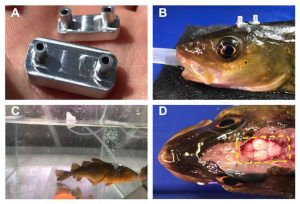
Figure 1. Design and attachment method of the brain coolers on Atlantic cod.
Results
- The authors made the following CTMAX measurements (Fig. 2):
- Control fish without brain-coolers: 25.82 ± 0.58°C
- Ambient fish with brain-coolers with ambient ramping-temperature water: 25.96 ± 0.36°C
- Cooled fish with brain-coolers with ice-cold water: 26.33 ± 0.49°C
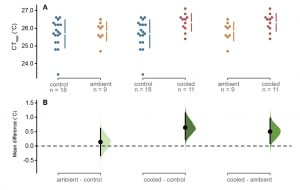
Figure 2. Critical thermal maximum measured as loss of equilibrium temperature in the three groups of Atlantic cod.
Discussion and Conclusion
- The small difference in CTMAX between control fish and ambient fish indicates that the brain-coolers had a minimal effect on CTMAX.
- The small increase in CTMAX of brain cooled fish (0.5–0.7°C), relative to the large cooling effect of the brain coolers (2–6°C depending on the brain region), indicates that compromised brain nerve function is not the sole mechanism underlying LOE at CTMAX. The authors suggest that peripheral neurons and muscles could potentially have very similar thermal limits as the brain and therefore also be involved in setting the CTMAX. The authors also note that insufficient tissue oxygen supply is likely not the principal mechanism underlying the LOE of cod at CTMAX as brain cooling would not have protected against cardiac collapse.
- The authors conclude that the marginally increased CTMAX in cooled fish supports the hypothesis that LOE at thermal extremes is linked to compromised brain nerve function.
Interest
- Compromised nerve function in fishes at thermal extremes was first proposed almost 50 years ago (e.g., Friedlander et al., 1976), but the findings have been largely forgotten because of the dominating role of the oxygen-limitation hypothesis in the field of fish ecophysiology. The study by Jutfelt et al. nicely bridges these early studies with the recent studies showing that fishes maintain tissue oxygen supply at thermal extremes.
Future directions
- Using externally mounted coolers (as in this study) or internally implanted thermodes, future studies aimed at identifying organs linked to the LOE in fishes at CTMAX could test if CTMAX is affected by localised cooling (or heating) of other organs and tissues, such as the heart.
- Future studies could also test if brain-cooling increases the CTMAX of crustaceans, as species from this group have also been shown to maintain tissue oxygen supply at thermal extremes.
- Finally, a 10°C h-1 ramping rate is high both from an environmental perspective and relative to other CTMAX studies on fishes. Future studies could test how reducing the temperature ramping rate affect the magnitude of brain-cooling on CTMAX.
References
- Friedlander MJ, Kotchabhakdi N, Prosser CL. 1976. Effects of cold and heat on behavior and cerebellar function in Goldfish. Comp. Physiol. 112, 19–45.
- Jutfelt F et al. 2018. Oxygen- and capacity-limited thermal tolerance: blurring ecology and physiology. Exp. Biol. 221, jeb169615. (doi: 10.1242/jeb. 169615)
- Pörtner HO. 2010. Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. Exp. Biol. 213, 881–893. (doi: 10.1242/jeb.037523)
- Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Clim. Change 2, 686-690 (doi: 10.1038/nclimate1539)
doi: https://doi.org/10.1242/prelights.11453
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the ecology category:
Gestational exposure to high heat-humidity conditions impairs mouse embryonic development
Girish Kale, preLights peer support
Blue appendages and temperature acclimation increase survival during acute heat stress in the upside-down jellyfish, Cassiopea xamachana
Maitri Manjunath
How the liver contributes to stomach warming in the endothermic white shark Carcharodon carcharias
Sarah Young-Veenstra
Also in the physiology category:
Gestational exposure to high heat-humidity conditions impairs mouse embryonic development
Girish Kale, preLights peer support
Modular control of time and space during vertebrate axis segmentation
AND
Natural genetic variation quantitatively regulates heart rate and dimension
Girish Kale, Jennifer Ann Black
Blue appendages and temperature acclimation increase survival during acute heat stress in the upside-down jellyfish, Cassiopea xamachana
Maitri Manjunath
preLists in the ecology category:
preLights peer support – preprints of interest
This is a preprint repository to organise the preprints and preLights covered through the 'preLights peer support' initiative.
| List by | preLights peer support |
EMBO | EMBL Symposium: The organism and its environment
This preList contains preprints discussed during the 'EMBO | EMBL Symposium: The organism and its environment', organised at EMBL Heidelberg, Germany (May 2023).
| List by | Girish Kale |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
Also in the physiology category:
Fibroblasts
The advances in fibroblast biology preList explores the recent discoveries and preprints of the fibroblast world. Get ready to immerse yourself with this list created for fibroblasts aficionados and lovers, and beyond. Here, my goal is to include preprints of fibroblast biology, heterogeneity, fate, extracellular matrix, behavior, topography, single-cell atlases, spatial transcriptomics, and their matrix!
| List by | Osvaldo Contreras |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
TAGC 2020
Preprints recently presented at the virtual Allied Genetics Conference, April 22-26, 2020. #TAGC20
| List by | Maiko Kitaoka et al. |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Cellular metabolism
A curated list of preprints related to cellular metabolism at Biorxiv by Pablo Ranea Robles from the Prelights community. Special interest on lipid metabolism, peroxisomes and mitochondria.
| List by | Pablo Ranea Robles |











 (No Ratings Yet)
(No Ratings Yet)