Brief sensory deprivation triggers cell type-specific structural and functional plasticity in olfactory bulb neurons
Posted on: 3 June 2020
Preprint posted on 12 May 2020
Article now published in The Journal of Neuroscience at http://dx.doi.org/10.1523/JNEUROSCI.1606-20.2020
Nasal congestion? Neurons in the olfactory bulb adapt their electrical activity upon nose occlusion
Selected by Ana Dorrego-RivasCategories: neuroscience
Background and introduction
In most neurons, action potentials are initiated at the axonal initial segment (AIS), a neuronal subcompartment located within the proximal axon (Leterrier, 2018; Kole and Stuart, 2012). Several studies have shown that the AIS is plastic and can change in length and/or position to regulate neuronal excitability (Grubb and Burrone, 2010). In vivo, the AIS undergoes structural changes in response to an imbalanced input, either by excess or by defect. AIS remodeling can occur during development and in the adult brain in response to different stimulus (Kuba et al., 2010; Gutzmann et al., 2014; Jamann et al., 2020). This kind of plasticity is complex: the AIS will respond differently to the same input depending on the brain region and neuron type. Computational simulations have predicted the output associated to different AIS responses considering morphological parameters, like the size of the neuron or the arborization complexity, but few works have addressed these findings in the brain (Gulledge and Bravo, 2016).
Chand and colleagues showed in 2015 that dopaminergic inhibitory neurons from the olfactory bulb in culture display particular AIS changes under treatment with tetrodotoxin (a drug for blocking sodium channels): while previous studies reported an AIS lengthening in deprivation conditions (Kuba et al., 2010), the AIS of these neurons was shorter. The following work describes the same AIS structural plasticity and a modulation of the electrical activity occurring in these neurons in vivo after short nasal occlusion.
Key findings
The study starts with the validation of a method for sensory deprivation based on a custom-made plug to occlude one of the mouse nostrils for 24h. The selected timing is short enough to potentially trigger adaptive responses without reaching a pathological context. By using non-plugged mice as controls, the study confirms that this method does not damage the olfactory epithelium (OE): the OE thickness and the number of OMP (marker for olfactory sensory neurons) and caspase-3 (marker for apoptotic cells) positive cells were not different between controls and plugged mice.
How do neurons in the olfactory bulb (OB) react to such sensory deprivation? The authors observed that, in dopaminergic (DA) inhibitory neurons, the fluorescence intensity of c-fos – a marker used to assess the level of neuronal activity – was decreased in the occluded animals when compared to their control counterparts. Previous work from the lab leading this study described two morphologically and functionally defined populations within OB DA neurons: axon/AIS-bearing cells and anaxonic neurons (Galliano et al., 2018). The staining of c-fos in both groups of cells was reduced in the sensory-deprived mice.
How are excitatory neurons responding to nasal occlusion? Both mitral/tufted cells (M/TCs) and external tufted cells (ETC) shown decreased levels of another activity marker, phospho-S6 ribosomal protein (pS6). At this level, both inhibitory and excitatory neurons are responding similarly. Knowing that the AIS displays activity-dependent structural plasticity, is nasal occlusion promoting such changes in neurons from the OB? No alteration in length and position was found in the AIS of M/TCs and ETCs (glutamatergic profile) and the intrinsic excitability of these cells in the sensory-deprived mice did not differ from the controls. Regarding inhibitory DA OB neurons, the authors found a decrease on the levels of tyrosine hydroxylase (TH, the enzyme for DA synthesis and marker of DA neurons) expression, but not of TH positive cells, upon nasal occlusion. Of note, the expression of TH in bulbar neurons is regulated by activity (Baker et al., 1983). These findings were true for the two populations of DA OB neurons, namely AIS/axon bearing cells and anaxonic neurons. Remarkably, the results provide evidence for the fastest activity-dependent TH change observed in this cell class in vivo: TH changes have been shown by many studies but usually with long-term manipulations. Whole-cell patch clamp recordings in anaxonic neurons revealed no differences in excitability between occluded mice and the controls, showing that the TH decrease does not correlate with a change in electrical activity in these cells.
Interestingly, the authors observed something particular in the AIS of the inhibitory DA OB axon-bearing neurons: while no difference in distance from the soma was found for both groups of animals, the AIS from sensory-deprived mice was shorter in comparison to the controls (Fig. 1). Although the passive membrane properties were similar between the two groups, this structural AIS change was associated with alterations in the intrinsic excitability of neurons from occluded mice: the action potential threshold (the current needed for generating a spike) was higher and the firing frequency was decreased.
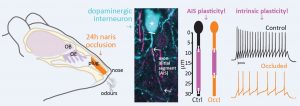
Figure 1: brief (24h) sensory deprivation via naris occlusion promotes the shortening of the AIS of axon-bearing DA OB neurons. Image from Galliano et al, 2020.
These results show that only 1 day of naris occlusion is enough to trigger AIS plasticity in axon-bearing DA OB neurons and an overall decrease of their intrinsic excitability. As mentioned previously, these neurons were found to react similarly in vitro: a decrease of activity, induced by exposure to tetrodotoxin for 24h, leads to a shortening of the AIS (Chand et al, 2015). The results of the present study confirm that this kind of plasticity occurs also in the brain.
In addition, this work shows that, from all the explored sets of cells, the brief sensory deprivation only promoted a change in these specific axon-bearing DA OB neurons. The also AIS-bearing M/TCs and ETCs, which are excitatory cells, show no AIS plasticity and no changes in electrical activity. The plasticity promoted via occlusion is, therefore, cell-type specific.
Why did I choose this preprint?
The field of AIS plasticity, especially in vivo, remains to be widely explored. Most of studies have shown activity-independent and dependent changes in AIS length and/or position in sensory systems: neurons from the nucleus magnocellularis (auditory), retinal ganglion cells (visual) and neurons from the somatosensory cortex (whisker-to-barrel system). This work is the first one showing this type of plasticity taking place in inhibitory neurons in vivo (and, particularly, within the olfactory bulb). The lab leading this study had shown previously that these specific OB DA inhibitory axon-bearing neurons displayed structural AIS plasticity in vitro. The present study confirms these findings in the brain and opens a path for further research on how this sensory deprivation can modulate brain networks within the OB and beyond. Apart from its scientific significance, this work is based on highly robust data that considers all the possible variability when analysing a system in vivo: for example, the co-embedding of control and occluded slices to assess intensity of staining.
References
Leterrier, C. (2018). The axonal initial segment: an updated viewpoint. J Neurosci, 38(9), 2135–2145
Kole, M.H., Stuart G.J. (2012). Signal processing in the axon initial segment. Neuron, 73(2), 235–247.
Grubb, M.S., and Burrone, J. (2010). Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070-1074.
Gutzmann, A., Ergul, N., Grossmann, R., Schultz, C., Wahle, P., and Engelhardt, M. (2014). A period of structural plasticity at the axon initial segment in developing visual cortex. Front Neuroanat 8, 11.
Kuba, H., Oichi, Y., and Ohmori, H. (2010). Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 465, 1075-1078.
Jamann, N., Dannehl, D., Wagener, R., Corcelli, C., Schultz, C., Staiger, J., Kole, M.H.P and Engelhardt, M. (2020). Sensory input drives rapid homeostatic scaling of the axon initial segment in the mouse barrel cortex. BioRxiv – https://doi.org/10.1101/2020.02.27.968065.
Gulledge, A.T. and Bravo, J.J. (2016). Neuron morphology influences axon initial segment plasticity. eNeuro, 3(1), ENEURO.0085-15.2016.
Chand, A.N., Galliano, E., Chesters, R.A. and Grubb, M.S. (2015). A distinct subtype of dopaminergic interneuron displays inverted structural plasticity at the axon initial segment. The Journal of Neuroscience 35, 4, 1573-90.
Galliano E., Franzoni E., Breton M., Chand A.N., Byrne D.J., Murthy V.N. and Grubb, M.S. (2018). Embryonic and postnatal neurogenesis produce functionally distinct subclasses of dopaminergic neurons. eLife 7, e32373.
Baker H., Kawano T., Margolis F.L. and Joh T.H. (1983) Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci 3: 69-78.
doi: https://doi.org/10.1242/prelights.20657
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the neuroscience category:
Imaging cellular activity simultaneously across all organs of a vertebrate reveals body-wide circuits
Muhammed Sinan Malik
Post-translational Tuning of Human Cortical Progenitor Neuronal Output
Jawdat Sandakly
Maturation of the glymphatic system confers innate resistance of the brain to Zika virus infection
Jimeng Li
preLists in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (1 votes)
(1 votes)