Experience-dependent plasticity of a highly specific olfactory circuit in Drosophila melanogaster
Posted on: 16 February 2024 , updated on: 17 February 2024
Preprint posted on 2 August 2023
How flies get used to the smell of death: new study shows brain changes and behavioral adaptation in Drosophila to a highly repulsive odor.
Selected by T. W. SchwanitzCategories: animal behavior and cognition, neuroscience
Introduction (how flies get used to the smell of death)
There are certain smells that set off alarm bells because they signal potential dangers. For human beings, one such smell is that of dead, putrefying carcasses. It is hard to ignore, and it just makes you want to escape. For fruit flies, an equivalent scent is the odorant geosmin. This chemical emanates from toxic molds, proving lethal to both flies and their larvae—so, when flies catch a whiff of it, they know it is time to make a quick escape.
Given the significance of this odorant to flies, extensive research has focused on the neural circuit governing its detection in Drosophila melanogaster: olfactory sensory neurons of a single type detect this chemical via the narrowly tuned odorant receptor Or56a. All Or56a-expressing olfactory sensory neurons converge in a singular spherical sub-region, known as a glomerulus, within the antennal lobe—the brain’s odor-processing region.
It was initially thought that the antennal lobe and the glomeruli in it were highly stereotyped and not altered by experience, in part because even drastic interventions such as removing all the neural inputs to this region did not result in equally drastic changes. Since then, however, numerous studies have shown that the fly’s experience can alter the volume of different glomeruli, suggesting that either the number of neurons or the number of synaptic connections they make with each other could change. Fabian and colleagues were therefore interested in understanding the effects of chronic geosmin exposure on the Or56a glomerulus, also known as the DA2 glomerulus (Fig. 1). For an odorant so critical to the fly’s survival, is it possible that their neural circuit is “soft-wired” instead of hard-wired—that is, might this glomerulus also change its volume or show other effects from odorant exposure?
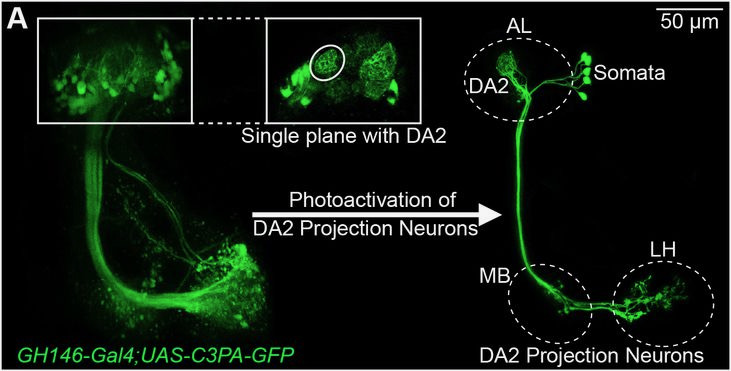
Fig. 1. Example image of the geosmin-sensing glomerulus (DA2) in the antennal lobe (AL) and projection neurons going to higher brain centers, the mushroom body (MB) and the lateral horn (LH). Images were created with D. melanogaster lines that express photoactivatable GFP in the projection neurons.
Highlighted results
First, the researchers confirmed that prolonged exposure to geosmin does indeed change the volume of the Or56a glomerulus. Adult flies were exposed to one-minute-long pulses of geosmin odor every five minutes over a span of four days—a considerable exposure and amount of time for fruit flies. These flies were expressing photoactivatable GFP, which made it possible to induce green fluorescence in specific neurons in the brain. The authors thus were able to show that the Or56a glomerulus gets larger in flies exposed to geosmin relative to control flies exposed to mineral oil.
The authors found the following circuit-level changes leading to this increase in volume:
- Projection neurons: the number of neurons going from the Or56a glomerulus to higher brain centers remained the same. These projection neurons did not show any detectable differences in their structure in higher brain centers, e.g., the mushroom body or the lateral horn. The number of axon terminals, boutons, and axon length all did not show any changes in the Or56a glomerulus projection neurons in higher brain centers. However, it appeared that projection neurons did expand their dendritic fields in the glomerulus itself within the antennal lobe.
- Local interneurons: the neurons that connect the Or56a glomerulus to other glomeruli in the antennal lobe showed an increase in volume also, but only for one out of the four local interneuron sub-populations examined. The increase observed in this line was largely due to an increase in local interneuron boutons, i.e., swellings along the neuronal branches with high synaptic densities. Other subpopulations showed varying effects. These results demonstrate that long term geosmin exposure had different effects on specific local interneuron subpopulations at the synaptic and morphological level.
- Olfactory sensory neurons and glial cells: The authors did not see an increase in olfactory sensory neuron or glial cell volume. However, the authors suspected that heightened activation of the olfactory sensory neurons might lead to mitochondrial changes in those neurons. Using a fly line with GFP tagged to mitochondria, the authors found evidence that, in olfactory sensory neurons, the number of mitochondria increases while their overall volume remains the same, suggesting higher levels of mitochondrial fission.
The authors also looked at other glomeruli that do not respond to geosmin and found that they did not change in the same way that the Or56a glomerulus did. Thus, only circuits that are activated by the exposure were modulated. Taken together with prior findings in the literature, these results suggest that chronic exposure to an odorant can cause that odorant’s glomerulus to get larger, or it could sometimes have the opposite effect. There seem to be general patterns and similarities in plasticity effects, yet no firm rules seem to exist (as far as we can tell right now).
Importantly, Fabian and colleagues demonstrated that the circuit changes they observed also translate to behavioral changes: on average, flies exposed long-term to geosmin are not as repelled by the odor, going so far as to tolerate it for feeding and oviposition—even though calcium imaging data suggests that these flies can still detect geosmin as well as control flies.
Why I liked this study
Given that the insect brain is often viewed as stereotyped due to early studies of the antennal lobe, it is nice to see a more recent antennal lobe study showing that there is greater plasticity and complexity in this brain region than previously imagined. This paper adds to a growing corpus of plasticity work. Although no firm rules regarding mechanisms for neural plasticity have been established yet, this type of study provides a necessary foundation for eventually developing them. It is also fascinating to see D. melanogaster flies acclimate to an odor that should be a major repellent. This gives me hope that I may yet get used to even the nastiest smells that I frequently encounter.
doi: https://doi.org/10.1242/prelights.36557
Read preprintSign up to customise the site to your preferences and to receive alerts
Register hereAlso in the animal behavior and cognition category:
Cannibalism as a mechanism to offset reproductive costs in three-spined sticklebacks
Tina Nguyen
Morphological variations in external genitalia do not explain the interspecific reproductive isolation in Nasonia species complex (Hymenoptera: Pteromalidae)
Stefan Friedrich Wirth
Trade-offs between surviving and thriving: A careful balance of physiological limitations and reproductive effort under thermal stress
Tshepiso Majelantle
Also in the neuroscience category:
PPARδ activation in microglia drives a transcriptional response that primes phagocytic function while countering inflammatory activation
Isabel Paine
The lipidomic architecture of the mouse brain
CRM UoE Journal Club et al.
Self-renewal of neuronal mitochondria through asymmetric division
Lorena Olifiers
preLists in the animal behavior and cognition category:
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
9th International Symposium on the Biology of Vertebrate Sex Determination
This preList contains preprints discussed during the 9th International Symposium on the Biology of Vertebrate Sex Determination. This conference was held in Kona, Hawaii from April 17th to 21st 2023.
| List by | Martin Estermann |
Bats
A list of preprints dealing with the ecology, evolution and behavior of bats
| List by | Baheerathan Murugavel |
FENS 2020
A collection of preprints presented during the virtual meeting of the Federation of European Neuroscience Societies (FENS) in 2020
| List by | Ana Dorrego-Rivas |
Also in the neuroscience category:
November in preprints – DevBio & Stem cell biology
preLighters with expertise across developmental and stem cell biology have nominated a few developmental and stem cell biology (and related) preprints posted in November they’re excited about and explain in a single paragraph why. Concise preprint highlights, prepared by the preLighter community – a quick way to spot upcoming trends, new methods and fresh ideas.
| List by | Aline Grata et al. |
October in preprints – DevBio & Stem cell biology
Each month, preLighters with expertise across developmental and stem cell biology nominate a few recent developmental and stem cell biology (and related) preprints they’re excited about and explain in a single paragraph why. Short, snappy picks from working scientists — a quick way to spot fresh ideas, bold methods and papers worth reading in full. These preprints can all be found in the October preprint list published on the Node.
| List by | Deevitha Balasubramanian et al. |
October in preprints – Cell biology edition
Different preLighters, with expertise across cell biology, have worked together to create this preprint reading list for researchers with an interest in cell biology. This month, most picks fall under (1) Cell organelles and organisation, followed by (2) Mechanosignaling and mechanotransduction, (3) Cell cycle and division and (4) Cell migration
| List by | Matthew Davies et al. |
July in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: (1) Cell Signalling and Mechanosensing (2) Cell Cycle and Division (3) Cell Migration and Cytoskeleton (4) Cancer Biology (5) Cell Organelles and Organisation
| List by | Girish Kale et al. |
May in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) Biochemistry/metabolism 2) Cancer cell Biology 3) Cell adhesion, migration and cytoskeleton 4) Cell organelles and organisation 5) Cell signalling and 6) Genetics
| List by | Barbora Knotkova et al. |
April in preprints – the CellBio edition
A group of preLighters, with expertise in different areas of cell biology, have worked together to create this preprint reading lists for researchers with an interest in cell biology. This month, categories include: 1) biochemistry/metabolism 2) cell cycle and division 3) cell organelles and organisation 4) cell signalling and mechanosensing 5) (epi)genetics
| List by | Vibha SINGH et al. |
Biologists @ 100 conference preList
This preList aims to capture all preprints being discussed at the Biologists @100 conference in Liverpool, UK, either as part of the poster sessions or the (flash/short/full-length) talks.
| List by | Reinier Prosee, Jonathan Townson |
2024 Hypothalamus GRC
This 2024 Hypothalamus GRC (Gordon Research Conference) preList offers an overview of cutting-edge research focused on the hypothalamus, a critical brain region involved in regulating homeostasis, behavior, and neuroendocrine functions. The studies included cover a range of topics, including neural circuits, molecular mechanisms, and the role of the hypothalamus in health and disease. This collection highlights some of the latest advances in understanding hypothalamic function, with potential implications for treating disorders such as obesity, stress, and metabolic diseases.
| List by | Nathalie Krauth |
‘In preprints’ from Development 2022-2023
A list of the preprints featured in Development's 'In preprints' articles between 2022-2023
| List by | Alex Eve, Katherine Brown |
CSHL 87th Symposium: Stem Cells
Preprints mentioned by speakers at the #CSHLsymp23
| List by | Alex Eve |
Journal of Cell Science meeting ‘Imaging Cell Dynamics’
This preList highlights the preprints discussed at the JCS meeting 'Imaging Cell Dynamics'. The meeting was held from 14 - 17 May 2023 in Lisbon, Portugal and was organised by Erika Holzbaur, Jennifer Lippincott-Schwartz, Rob Parton and Michael Way.
| List by | Helen Zenner |
ASCB EMBO Annual Meeting 2019
A collection of preprints presented at the 2019 ASCB EMBO Meeting in Washington, DC (December 7-11)
| List by | Madhuja Samaddar et al. |
SDB 78th Annual Meeting 2019
A curation of the preprints presented at the SDB meeting in Boston, July 26-30 2019. The preList will be updated throughout the duration of the meeting.
| List by | Alex Eve |
Autophagy
Preprints on autophagy and lysosomal degradation and its role in neurodegeneration and disease. Includes molecular mechanisms, upstream signalling and regulation as well as studies on pharmaceutical interventions to upregulate the process.
| List by | Sandra Malmgren Hill |
Young Embryologist Network Conference 2019
Preprints presented at the Young Embryologist Network 2019 conference, 13 May, The Francis Crick Institute, London
| List by | Alex Eve |











 (1 votes)
(1 votes)